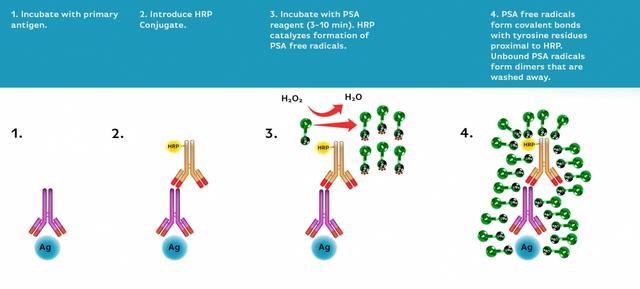
Biotin Styramide *Superior Replacement for Biotin Tyramide*
Example protocol
AT A GLANCE
- Fix/permeabilize/block cells or tissue
- Add primary antibody in blocking buffer
- Add HRP-conjugated secondary antibody
- Prepare Styramide™ working solution and apply in cells or tissue for 5-10 minutes at room temperature
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Add 100 µL of DMSO into the vial of Biotin-labeled Styramide™ conjugate to make 100X Styramide™ stock solution.
Note: Make single-use aliquots and store unused 100X stock solution at -20 °C in a dark place. Avoid repeat freeze-thaw cycles.
Add 10 µL of 3% hydrogen peroxide (not provided) to 90 µL of ddH2O.
Note: Prepare the 100X H2O2 solution fresh on the day of use.
PREPARATION OF WORKING SOLUTION
Every 1 mL of Reaction Buffer requires 10 µL of Styramide™ stock solution and 10 µL of H2O2 stock solution.
Note: The Styramide™ provided is enough for 100 tests based on 100 µL of Styramide™ working solution needed per coverslip or per well in a 96-well microplate.
Note: The Styramide™ working solution must be used within 2 hours after preparation and avoid direct exposure to light.
Make appropriate concentration of secondary antibody-HRP working solution as per the manufacturer's recommendations.
SAMPLE EXPERIMENTAL PROTOCOL
This protocol is applicable for both cells and tissues staining.
- Fix the cells or tissue with 3.7% formaldehyde or paraformaldehyde, in PBS at room temperature for 20 minutes.
- Rinse the cells or tissue with PBS twice.
- Permeabilize the cells with 0.1% Triton X-100 solution for 1-5 minutes at room temperature.
- Rinse the cells or tissue with PBS twice.
Deparaffinize and dehydrate the tissue according to the standard IHC protocols. Perform antigen retrieval with the preferred specific solution/protocol as needed. A protocol can be found at:
https://www.aatbio.com/resources/guides/paraffin-embedded-tissue-immunohistochemistry-protocol.html
Optional: Quench endogenous peroxidase activity by incubating cell or tissue sample in peroxidase quenching solution (such as 3% hydrogen peroxide) for 10 minutes. Rinse with PBS twice at room temperature.
Optional: If using HRP-conjugated streptavidin, it is advisable to block endogenous biotins by biotin blocking buffer.
- Block with preferred blocking solution (such as PBS with 1% BSA) for 30 minutes at 4 °C.
- Remove blocking solution and add primary antibody diluted in recommended antibody diluent for 60 minutes at room temperature or overnight at 4 °C.
- Wash with PBS three times for 5 minutes each.
Apply 100 µL of the secondary antibody-HRP working solution to each sample and incubate for 60 minutes at room temperature.
Note: Incubation time and concentration can be varied depending on the signal intensity.
- Wash with PBS three times for 5 minutes each.
Prepare and apply 100 µL of Styramide™ working solution to each sample and incubate for 5-10 minutes at room temperature.
Note: If you observe a non-specific signal, you can shorten the incubation time with Styramide™. You should optimize the incubation period using positive and negative control samples at various incubation time points. Or you can use a lower concentration of Styramide™ in the working solution.- Rinse with PBS three times.
- Counterstain the cell or tissue samples as needed. AAT provides a series of nucleus counterstain reagents as listed in Table 1. Follow the instruction provided with the reagents.
Mount the coverslip using a mounting medium with anti-fading properties.
Note: To ensure optimal results, it is recommended to use either ReadiUse™ microscope mounting solution (Cat. 20009) or FluoroQuest™ TSA/PSA Antifade Mounting Medium *Optimized for Tyramide and Styramide Imaging* (Cat. 44890) instead of Vectashield® mounting media. There are instances where Vectashield® mounting media may not be suitable for certain TSA/PSA conjugates.
- Use the appropriate filter set to visualize the signal from the Styramide labeling.
Table 1. Products recommended for nucleus counterstain.
| Cat# | Product Name | Ex/Em (nm) |
| 17548 | Nuclear Blue™ DCS1 | 350/461 |
| 17550 | Nuclear Green™ DCS1 | 503/526 |
| 17551 | Nuclear Orange™ DCS1 | 528/576 |
| 17552 | Nuclear Red™ DCS1 | 642/660 |
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 129.879 µL | 649.393 µL | 1.299 mL | 6.494 mL | 12.988 mL |
| 5 mM | 25.976 µL | 129.879 µL | 259.757 µL | 1.299 mL | 2.598 mL |
| 10 mM | 12.988 µL | 64.939 µL | 129.879 µL | 649.393 µL | 1.299 mL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |