Cell Meter™ Fluorimetric Cellular Lipid Peroxidation Assay Kit
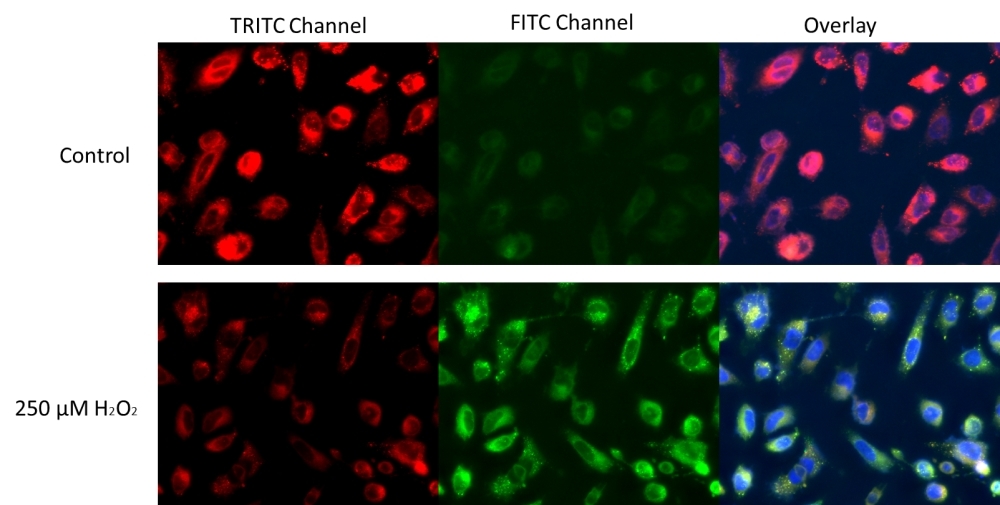
Lipid peroxidation is the oxidative degradation of cellular lipid by reactive oxygen species (ROS). This process can lead to not only disruption of the cellular membrane integrity, but also inactivation of membrane-bound receptors. It is one of the main causes of free radical-mediated damages in cells. Cell Meter™ Fluorimetric Cellular Lipid Peroxidation Assay Kit provides a sensitive method for monitoring lipid peroxidation. The kit uses our sensitive ratiometric lipid peroxidation sensor, Lipoxite™ R590/G520 that changes its red fluorescence from red to green upon peroxidation by ROS in cells, this peroxidation-dependent shift enables the ratiometric measurement of lipid peroxidation. Our kit includes H2O2 as a positive control treatment to induce lipid peroxidation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22906 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm, 575/26 nm filter |
| Instrument specification(s) | FITC channel |
| Fluorescence microscope | |
| Excitation | 490 nm (FITC) and 545 nm (TRITC) |
| Emission | 530 nm (FITC) and 600 nm (TRITC) |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | FITC and TRITC channels |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 19, 2026
