CellPaint™ TSP membrane stain
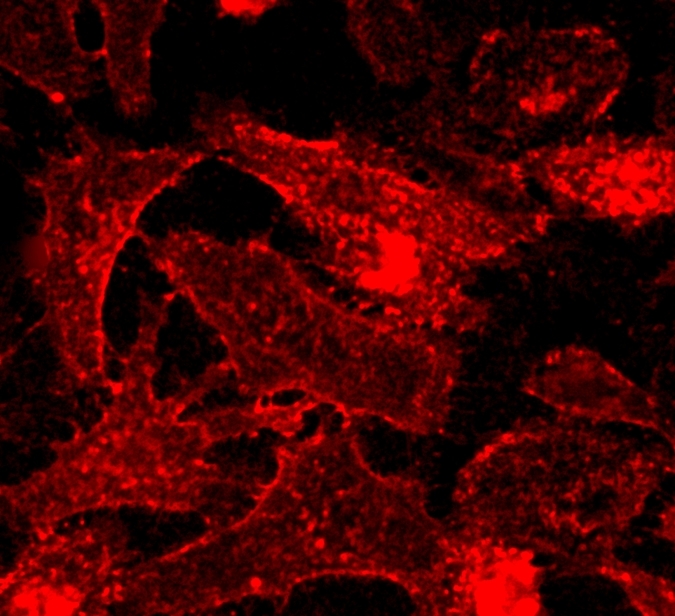
TSP is a styrylpyridine-based fluorescent membrane probe suitable for imaging plasma membranes in living cells and tissues. It was reported by Guo et al in 2016 (Analyst, 2016, 141, 3228). The probe is a molecular rotor that has fluorescence sharply enhanced in viscous media. Its fluorescence is also microenvironment-sensitive, enables the turn-on imaging of plasma membranes with a high signal-to-noise ratio. Guo et al has demonstrated that TSP has high photostability, low cytotoxicity and excellent biocompatibility. It can also be used in 2 photon imaging.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22700 | 500 Tests | Price |
Physical properties
| Molecular weight | 1016.27 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 496 |
| Emission (nm) | 633 |
Storage, safety and handling
| Intended use | Research Use Only (RUO) |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Instrument settings
| Fluorescence microscope | |
| Excitation | Cy3/TRTC filter set |
| Emission | Cy3/TRITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 5, 2026

