FluoroQuest™ Anti-fading Kit I
Optimized for Slide Imaging
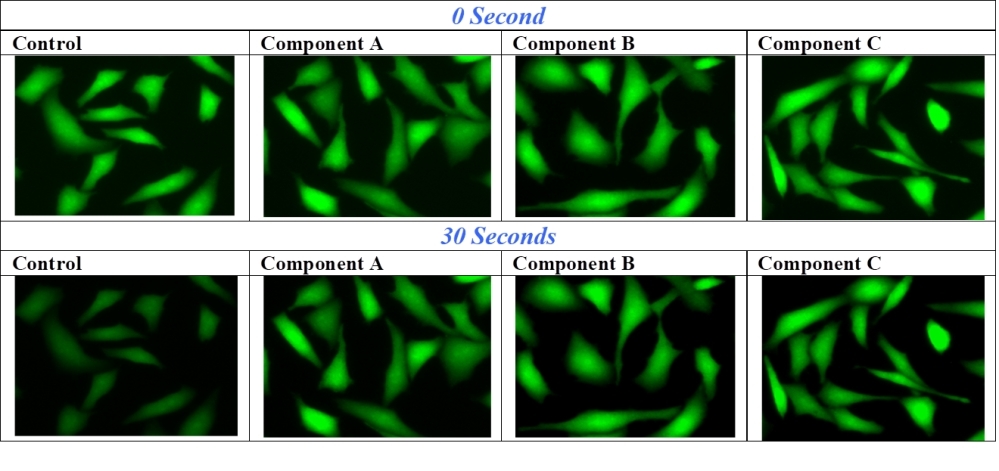
When exposed to excitation light, fluorescence intensity of dyes decreases due to their photooxidation or other photoreactions. There are very few fluorescent dyes that completely resist photobleaching. Frequently, when a section has been scanned repeatedly under strong excitation light, dyes could lose significant fluorescence signal before visual evaluation or photography can be accomplished. For examples, the photobleaching of fluoresceins (such as FITC-labeled antibodies) has become a major problem in fluorescence microscopy. In severe cases (such as phycoprotein-labeled bioconjugates), a fluorescence image of high resolution can not even be taken due to the extremely high photobleaching rate. Fluoroquest™ Anti-Fading Kit is to reduce the dye photobleaching rate, giving researchers longer observation time. The kit contains all the essential components that can be readily applied to imaging experiments. They are all premixed and ready-to-use solutions. This kit is designed for slide format while #20003 is designed for microplate format.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20001 | 1 kit | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | Varies |
| Emission | Varies |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 7, 2026
