MMP Green™ substrate
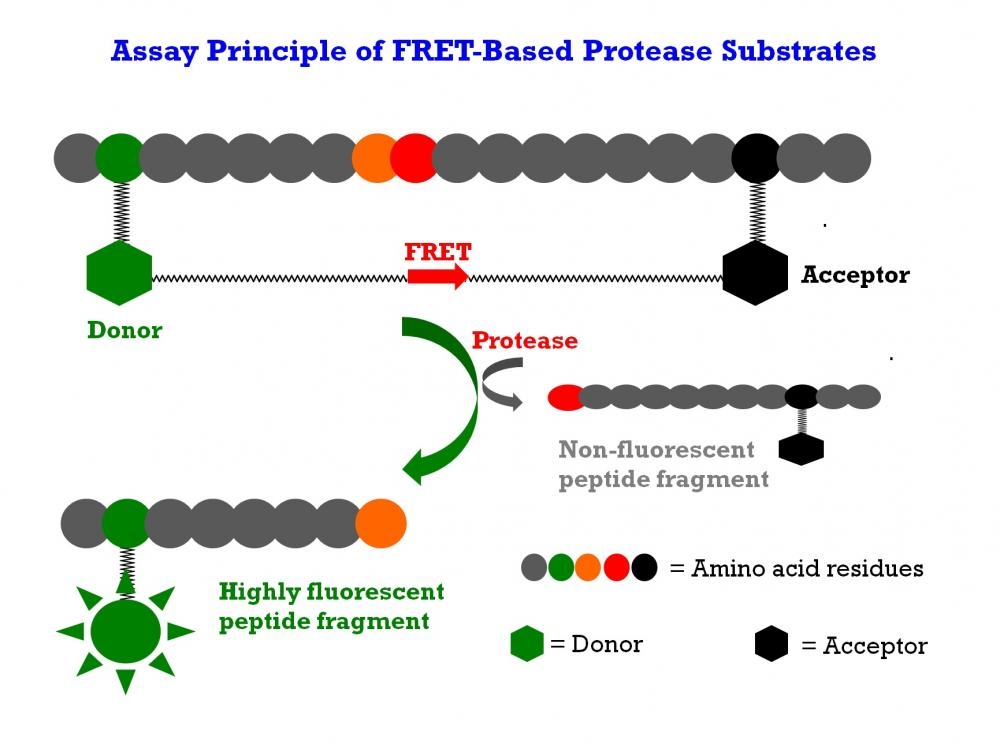
The matrix metalloproteinases (MMPs) constitute a family of zinc-dependent endopeptidases that function within the extracellular matrix. These enzymes are responsible for the breakdown of connective tissues and are important in bone remodeling, the menstrual cycle and repair of tissue damage. While the exact contribution of MMPs to certain pathological processes is difficult to assess, MMPs appear to play a key role in the development of arthritis as well as in the invasion and metastasis of cancer. MMPs tend to have multiple substrates, with most family members having the ability to degrade different types of collagen along with elastin, gelatin and fibronectin. It is quite difficult to find a substrate that is selective to a single MMP enzyme. This FRET substrate is designed to monitor the general activity of a MMP enzyme. It can also be used to screening MMP inhibitors when a purified MMP enzyme is used. This FRET substrate is based on our TF2/TQ2 FRET pair. Upon MMP hydrolysis the fluorescence of MMP Green™ FRET peptide substrate is increased since the TF2/TQ2 FRET pair is separated. The fluorescence increase is proportional to the MMP enzyme activities.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13519 | 100 Tests | Price | |
| 13520 | 1 mg | Price |
Physical properties
| Molecular weight | ~2000 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 494 |
| Emission (nm) | 515 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026

