Protonex™ Red 600-Zymosan A Conjugate
Product key features
The Protonex™ Red 600-Zymosan A Conjugate provides a ready-to-use, pH-sensitive fluorescent tool for monitoring phagocytosis and intracellular acidification processes in live cells.
- pH-activated fluorescence: Non-fluorescent at neutral pH and becomes strongly fluorescent in acidic compartments such as phagosomes and phagolysosomes.
- Zymosan-based targeting: Derived from yeast cell walls, Zymosan A is a potent biological substrate for phagocytic uptake.
- Multipurpose reagent: Suitable for incorporation into custom assay workflows for microscopy, flow cytometry, or plate-based readouts.
Product description
The Protonex™ Red 600–Zymosan A Conjugate is a ready-to-use reagent designed to study phagocytosis and phagosome acidification in live cells. This conjugate combines Zymosan A particles, a biologically active yeast cell wall component, with Protonex™ Red 600, a novel pH-sensitive fluorophore that remains non-fluorescent at neutral pH and becomes highly fluorescent upon entering acidic environments such as maturing phagosomes and phagolysosomes.
As a standalone reagent, it enables users to integrate phagocytic detection into their own custom assays. The TRITC-like excitation/emission properties of Protonex™ Red 600 make it compatible with a wide range of fluorescence imaging and detection systems. These conjugates can be combined with green fluorescent dyes such as GFP, Calbryte™ 520, calcein AM, or FITC-labeled antibodies to enable multiplexed analysis of cell function and viability. It is ideal for immunological research, drug discovery, and mechanistic studies of innate immune function, autophagy, or particle uptake.
Example protocol
AT A GLANCE
- Plate the cells.
- Treat cells with test compounds.
- Add Protonex Dye Zymosan A conjugates in medium.
- Incubate at 37°C for 60 minutes.
- Monitor fluorescence by microscope or fluorescence plate reader.
CELL PREPARATION
For guidelines on cell sample preparation, please visit:
https://www.aatbio.com/resources/guides/cell-sample-preparation.html
- Plate cells overnight in a growth medium at 20,000-50,000 cells/well/100 µL in a 96-well plate.
Note: For RAW 264.7 cells used in this assay, we recommend plating 50,000 cells per well in 100 µL of medium in a 96-well plate and incubating them overnight. It is important to optimize the cell density for each cell line individually.
Note: Higher background fluorescence levels may be seen with poly-D-lysine coated microplates.
SAMPLE EXPERIMENTAL PROTOCOL
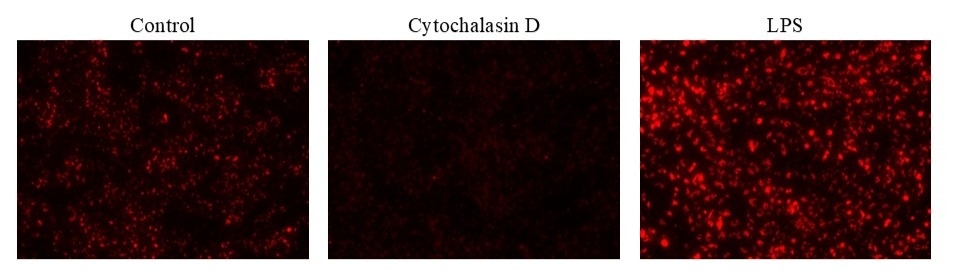
Add phagocytosis inhibitor or inducer (e.g., Cytochalasin D or LPS) at the desired concentrations. You may need to add vehicle controls to untreated wells. (For example: 11X working solution can be prepared in PBS, and 10 µL can be added to each well.)
Note: The time and concentration of phagocytosis effectors varies with cell types.
- Add the suspension of Zymosan A conjugate to the cell culture microplate in a 1:10 dilution, or 10 μL of particles added to 100 μL of cell culture medium, and mix well.
- Place the cells at 37°C for 60 minutes to 3 hours.
- Wash the cells 2-3 times with HHBS Buffer (AAT Cat# 20011) or buffer of your choice.
- Add 100 µL HHBS Buffer to each well.
- Observe plate with a fluorescence microscope using the following filter set or read plate in a fluorescence plate reader with bottom read mode.
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) |
| Protonex™ Red 670-Zymosan A Conjugate | 643 | 660 |
References
Authors: Liu, Wei and Liu, Bo
Journal: Methods in molecular biology (Clifton, N.J.) (2025): 171-175
Authors: Agina, Onyinyechukwu Ada and Shaari, Mohd Rosly and Isa, Nur Mahiza Md and Ajat, Mohd Mokrish Mohd and Zamri-Saad, Mohd and Samad, Mohd Jamil and Hamzah, Hazilawati
Journal: Research in veterinary science (2023): 105073
Authors: Piszczek, P and Wojcik-Piotrowicz, K and Nowak, B and Guzdek, P and Novak, P and Pytko-Polonczyk, J and Gil, K and Kaszuba-Zwoinska, J
Journal: Journal of physiology and pharmacology : an official journal of the Polish Physiological Society (2023)
Authors: Pavlova, Ekaterina and Shaposhnikova, Daria and Petrichuk, Svetlana and Radygina, Tatiana and Erokhina, Maria
Journal: Methods in molecular biology (Clifton, N.J.) (2023): 203-215
Authors: Tram, Trinh T B and Ha, Vu T N and Thu, Do D A and Dinh, Tran D and Vijay, Srinivasan and Hai, Hoang T and Hanh, Nguyen T and Phu, Nguyen H and Thwaites, Guy E and Thuong, Nguyen T T
Journal: Biology of the cell (2019): 262-270