Rhod-4™, sodium salt
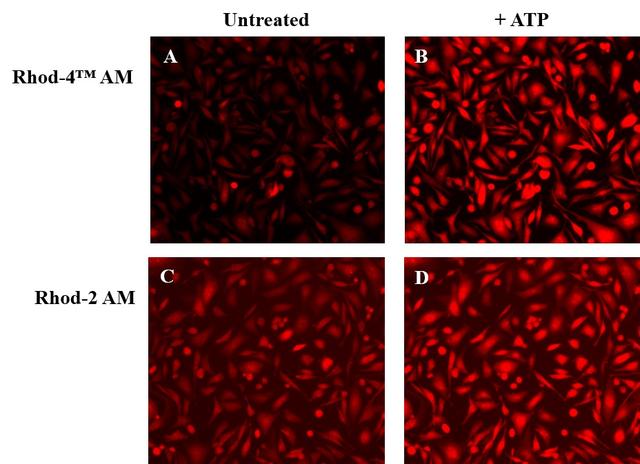
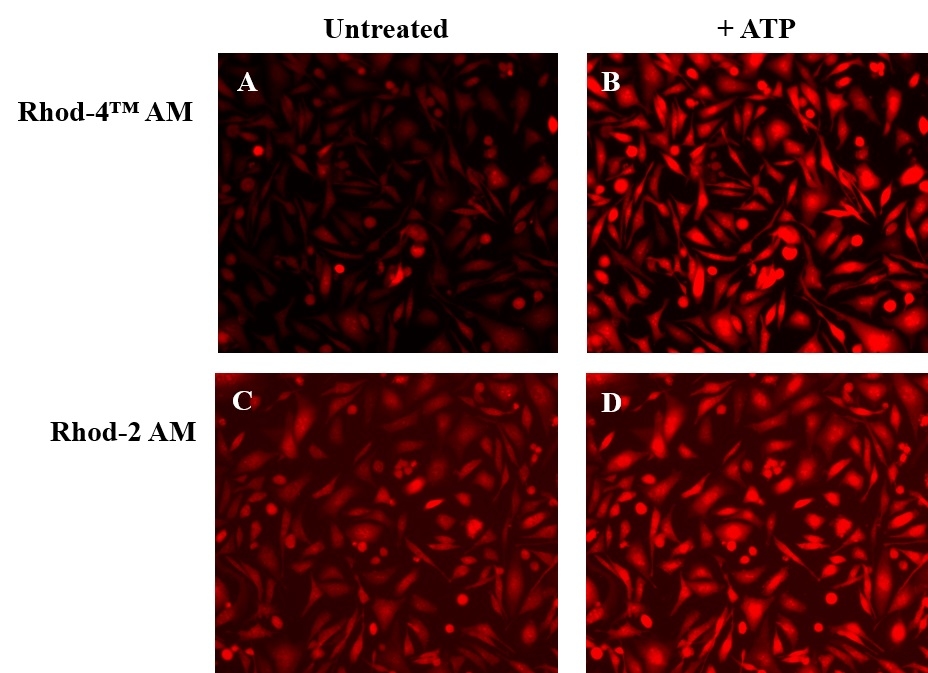
Calcium measurement is critical for numerous biological investigations. Fluorescent probes that show spectral responses upon binding Ca2+ have enabled researchers to investigate changes in intracellular free Ca2+ concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. Rhod-2 is most commonly used among the red fluorescent calcium indicators. However, Rhod-2 AM is only moderately fluorescent in live cells upon esterase hydrolysis, and has very small cellular calcium responses. Rhod-4™ has been developed to improve Rhod-2 cell loading and calcium response while maintaining the spectral wavelength of Rhod-2. In CHO and HEK cells Rhod-4™ AM has cellular calcium response that is 10 times more sensitive than Rhod-2 AM. AAT Bioquest offers versatile packing sizes of Quest Rhod-4 to meet your special needs, e.g., 1 mg; 10x50 µg; 20x50 µg; HTS packages with no additional packaging charge.
Calculators
Common stock solution preparation
Table 1. Volume of Water needed to reconstitute specific mass of Rhod-4™, sodium salt to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 122.603 µL | 613.016 µL | 1.226 mL | 6.13 mL | 12.26 mL |
| 5 mM | 24.521 µL | 122.603 µL | 245.206 µL | 1.226 mL | 2.452 mL |
| 10 mM | 12.26 µL | 61.302 µL | 122.603 µL | 613.016 µL | 1.226 mL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Quantum yield |
| Rhod-4™, potassium salt | 523 | 551 | 0.11 |
Citations
View all 23 citations: Citation Explorer
Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of inducedpluripotent stem (iPS) cells at different stages
Authors: Hirata, Mitsuhi and Yamaoka, Tetsuji
Journal: Acta Biomaterialia (2017)
Authors: Hirata, Mitsuhi and Yamaoka, Tetsuji
Journal: Acta Biomaterialia (2017)
Emerin plays a crucial role in nuclear invagination and in the nuclear calcium transient
Authors: Shimojima, Masaya and Yuasa, Shinsuke and Motoda, Chikaaki and Yozu, Gakuto and Nagai, Toshihiro and Ito, Shogo and Lachmann, Mark and Kashimura, Shin and Takei, Makoto and Kusumoto, Dai and others, undefined
Journal: Scientific Reports (2017)
Authors: Shimojima, Masaya and Yuasa, Shinsuke and Motoda, Chikaaki and Yozu, Gakuto and Nagai, Toshihiro and Ito, Shogo and Lachmann, Mark and Kashimura, Shin and Takei, Makoto and Kusumoto, Dai and others, undefined
Journal: Scientific Reports (2017)
Preliminary findings on ultrasound modulation of the electromechanical function of human stem-cell-derived cardiomyocytes
Authors: Chen, Andrew William and Klimas, Aleks and ra , undefined and Zderic, Vesna and Castellanos, Ivan Suares and Entcheva, Emilia
Journal: (2017): 1--4
Authors: Chen, Andrew William and Klimas, Aleks and ra , undefined and Zderic, Vesna and Castellanos, Ivan Suares and Entcheva, Emilia
Journal: (2017): 1--4
The role of spatial organization of Ca (2+) release sites in the generation of arrhythmogenic diastolic Ca (2+) release in myocytes from failing hearts.
Authors: Belevych, Andriy E and Ho, Hsiang-Ting and Bonilla, Ingrid M and Terentyeva, Radmila and Schober, Karsten E and Terentyev, Dmitry and Carnes, Cynthia A and Györke, Sándor
Journal: Basic research in cardiology (2017): 44
Authors: Belevych, Andriy E and Ho, Hsiang-Ting and Bonilla, Ingrid M and Terentyeva, Radmila and Schober, Karsten E and Terentyev, Dmitry and Carnes, Cynthia A and Györke, Sándor
Journal: Basic research in cardiology (2017): 44
Individual evaluation of cardiac marker expression and self-beating during cardiac differentiation of P19CL6 cells on different culture substrates
Authors: Yamaoka, Tetsuji and Hirata, Mitsuhi and Dan, Takaaki and Yamashita, Atsushi and Otaka, Akihisa and Nakaoki, Takahiko and Miskon, Azizi and Kakinoki, Sachiro and Mahara, Atsushi
Journal: Journal of Biomedical Materials Research Part A (2016)
Authors: Yamaoka, Tetsuji and Hirata, Mitsuhi and Dan, Takaaki and Yamashita, Atsushi and Otaka, Akihisa and Nakaoki, Takahiko and Miskon, Azizi and Kakinoki, Sachiro and Mahara, Atsushi
Journal: Journal of Biomedical Materials Research Part A (2016)
Page updated on August 9, 2025