Screen Quest™ Fura-2 No Wash Calcium Assay Kit
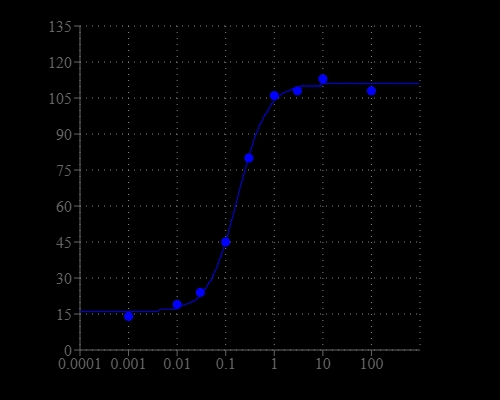
Calcium flux assays are preferred methods in drug discovery for screening G protein coupled receptors (GPCR). This ratiometric calcium assay kit allows homogeneous measurement of intracellular calcium changes caused by activation of G-protein-coupled receptors or calcium channels. Cells expressing a GPCR of interest that signals through calcium are pre-loaded with Fura-2 AM which can cross cell membrane. Once inside the cell, the lipophilic blocking groups of Fura-2 AM are cleaved by esterases, resulting in a negatively charged fluorescent dye that stays inside cells and its fluorescence wavelength is blue-shifted upon binding to calcium. When cells stimulated with agonists, the receptor signals the release of intracellular calcium, which greatly increase the fluorescence intensity of Fura-2 at the short wavelength. The ratiometric characteristics of Fura-2 make this kit an ideal tool for more accurate measurement of cellular calcium concentration compared to Fluo-4 of the single wavelength. With a single addition, the assay is easy to perform and desirable in a high thoroughput environment. The assay can be used in a convenient 96-well or 384-well microtiter-plate format and readily adapted to automation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36320 | 10 Plates | Price | |
| 36321 | 100 Plates | Price |
Spectral properties
| Excitation (nm) | 336 |
| Emission (nm) | 505 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 340/380 nm |
| Emission | 510 nm |
| Cutoff | 470 nm |
| Recommended plate | Black wall/Clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
| Other instruments | FDSS, FLIPR, FlexStation |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026

