TMRE
Tetramethylrhodamine ethyl ester; CAS#: 115532-52-0
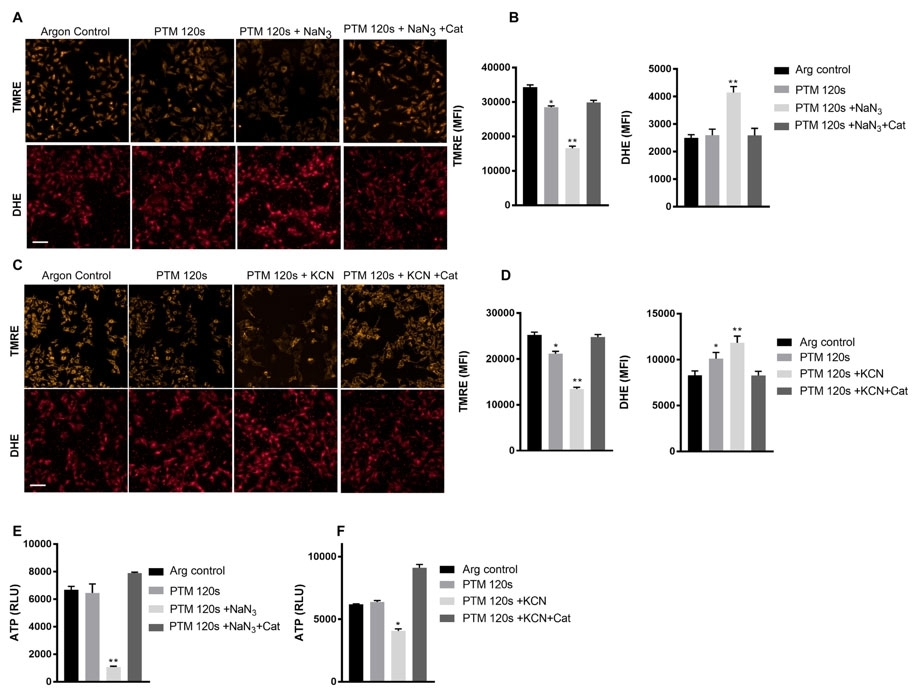
Positively charged rhodamine dyes (such as rhodamine esters and rosamines) are selectively localized in mitochondria, thus they are widely used for labeling mitochondria of live cells. Like JC-1, TMRM and TMRE are widely used for measuring mitochondrial membrane potential besides their selective mitochondrial staining. These two particular rhodamine esters stain mitochondria orange in fluorescence. Their spectral properties are similar to those of TRITC, making the use of TMRM and TMRE quite convenient. TMRE is slightly more hydrophobic than TMRM.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22220 | 25 mg | Price |
Physical properties
| Molecular weight | 514.95 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.27 |
| Correction factor (280 nm) | 0.03 |
| Excitation (nm) | 552 |
| Emission (nm) | 574 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| CAS | 115532-52-0 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

