Protonex™ Red 600 PEG12 maleimide
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | 1577.83 |
| Solvent | DMSO |
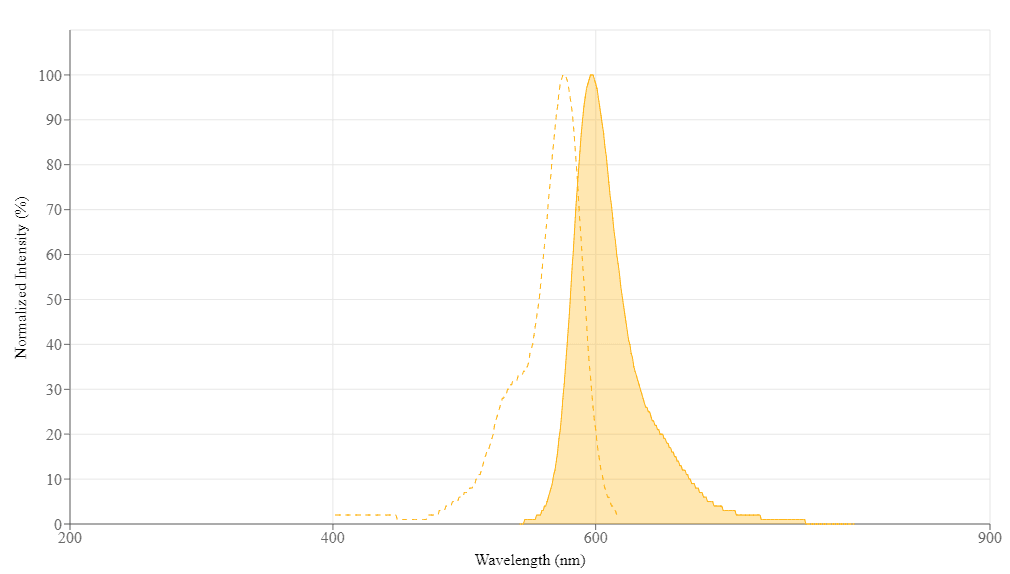
| Excitation (nm) | 576 |
| Emission (nm) | 597 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| Overview |
Molecular weight 1577.83 | Excitation (nm) 576 | Emission (nm) 597 |
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Mix 100 µL of a reaction buffer (e.g., 1 M sodium carbonate solution or 1 M phosphate buffer with pH ~9.0) with 900 µL of the target protein solution (e.g., antibody, protein concentration >2 mg/mL if possible) to give a 1 mL protein labeling stock solution.
Note: The pH of the protein solution (Solution A) should be 8.5 ± 0.5. If the pH of the protein solution is lower than 8.0, adjust the pH to the range of 8.0-9.0 using 1 M sodium bicarbonate solution or 1 M pH 9.0 phosphate buffer.
Note: The protein should be dissolved in 1X phosphate-buffered saline (PBS), pH 7.2-7.4. If the protein is dissolved in Tris or glycine buffer, it must be dialyzed against 1X PBS, pH 7.2-7.4, to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well. The presence of sodium azide or thimerosal might also interfere with the conjugation reaction. Sodium azide or thimerosal can be removed by dialysis or spin column for optimal labeling results.
Note: The conjugation efficiency is significantly reduced if the protein concentration is less than 2 mg/mL. For optimal labeling efficiency, the final protein concentration range of 2-10 mg/mL is recommended.
If your protein does not contain a free cysteine, you must treat your protein with DTT or TCEP to generate a thiol group. DTT or TCEP converts disulfide bonds to two free thiol groups. If you use DTT, you must remove free DTT by dialysis or gel filtration before conjugating the Protonex™ Red 600 PEG12 maleimide to your protein. The following is a sample protocol for generating a free thiol group:
Prepare a fresh solution of 1 M DTT (15.4 mg/100 µL) in distilled water.
To make an IgG solution in 20 mM DTT, add 20 µL of DTT solution per 1 mL of IgG solution while mixing well. Allow the solution to stand at room temperature for 30 minutes without additional mixing to reduce the oxidation of cysteines to cystines.
Pass the reduced IgG through the filtration column that has been pre-equilibrated with "Exchange Buffer." Collect 0.25 mL fractions from the column.
Determine the protein concentrations and pool the fractions with the majority of the IgG. This can be done either spectrophotometrically or colorimetrically.
It is recommended to carry out the conjugation immediately after this step. Please refer to the Sample Experiment Protocol for more details.
Note: IgG solutions should be >4 mg/mL for the best results. The protein should be concentrated if less than 2 mg/mL. An additional 10% should be included for losses on the buffer exchange column.
Note: The reduction can be carried out in almost any buffers from pH 7-7.5 (e.g., MES, phosphate, or TRIS buffers).
Note: Steps 3 and 4 can be replaced by dialysis.
Add anhydrous DMSO into the vial of Protonex™ Red 600 PEG12 maleimide to make a 10 mM stock solution. Mix well by pipetting or vortex.
Note: Prepare the dye stock solution (Solution B) before starting the conjugation. Use promptly. Extended storage of the dye stock solution may reduce the dye activity. Solution B can be stored in the freezer for two weeks when kept from light and moisture. Avoid freeze-thaw cycles.
SAMPLE EXPERIMENTAL PROTOCOL
This protocol was developed for conjugating Protonex™ Red 600 PEG12 maleimide to goat anti-mouse IgG. Additional optimization may be necessary for your specific protein.
Note: Each protein requires a distinct dye/protein ratio, which also depends on the properties of dyes. Over-labeling of a protein could detrimentally affect its binding affinity, while the protein conjugates of low dye/protein ratio give reduced sensitivity.
Use a 10:1 molar ratio of Solution B (dye)/Solution A (protein) as the starting point: Add 5 µL of the dye stock solution (Solution B, assuming the dye stock solution is 10 mM) into the vial of the protein solution (95 µL of Solution A) with effective shaking. The concentration of the protein is ~0.05 mM assuming the protein concentration is 10 mg/mL and the molecular weight of the protein is ~200 KD.
Note: We recommend using a 10:1 molar ratio of Solution B (dye)/Solution A (protein). If it is too low or too high, determine the optimal dye/protein ratio at 5:1, 15:1, and 20:1, respectively.
Continue to rotate or shake the reaction mixture at room temperature for 30-60 minutes.
The following protocol is an example of dye-protein conjugate purification by using a Sephadex G-25 column.
Prepare the Sephadex G-25 column according to the manufacturer's instructions.
Load the reaction mixture (From "Run conjugation reaction") to the top of the Sephadex G-25 column.
Add PBS (pH 7.2-7.4) as soon as the sample runs just below the top resin surface.
Add more PBS (pH 7.2-7.4) to the desired sample to complete the column purification. Combine the fractions that contain the desired dye-protein conjugate.
Note: For immediate use, the dye-protein conjugate needs to be diluted with staining buffer, and aliquoted for multiple uses.
Note: For longer-term storage, the dye-protein conjugate solution needs to be concentrated or freeze-dried.
The Degree of Substitution (DOS) is the most important factor for characterizing dye-labeled protein. Proteins of lower DOS usually have weaker fluorescence intensity, but proteins of higher DOS (e.g. DOS > 6) tend to have reduced fluorescence too. The optimal DOS for most antibodies is recommended between 2 and 10 depending on the properties of dye and protein. For effective labeling, the degree of substitution should be controlled to have 6-8 moles of Protonex™ Red 600 PEG12 maleimide to one mole of antibody. The following steps are used to determine the DOS of Protonex™ Red 600 PEG12 maleimide labeled proteins.
To measure the absorption spectrum of a dye-protein conjugate, it is recommended to keep the sample concentration in the range of 1-10 µM depending on the extinction coefficient of the dye.
For most spectrophotometers, the sample (from the column fractions) needs to be diluted with de-ionized water so that the OD values are in the range of 0.1 to 0.9. The O.D. (absorbance) at 280 nm is the maximum absorption of protein while 576 nm is the maximum absorption of Protonex™ Red 600 PEG12 maleimide. To obtain accurate DOS, make sure that the conjugate is free of the non-conjugated dye.
You can calculate DOS using our tool by following this link:
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 63.378 µL | 316.891 µL | 633.782 µL | 3.169 mL | 6.338 mL |
| 5 mM | 12.676 µL | 63.378 µL | 126.756 µL | 633.782 µL | 1.268 mL |
| 10 mM | 6.338 µL | 31.689 µL | 63.378 µL | 316.891 µL | 633.782 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
References
Authors: Fang, Liang and Li, Junying and Cheng, Hongyan and Liu, Huanhuan and Zhang, Caiyun
Journal: International journal of pharmaceutics (2024): 123854
Authors: Silswal, Akshay and Pramanik, Anup and Koner, Apurba Lal
Journal: Journal of materials chemistry. B (2024): 489-499
Authors: Briolay, Tina and Fresquet, Judith and Meyer, Damien and Kerfelec, Brigitte and Chames, Patrick and Ishow, Eléna and Blanquart, Christophe
Journal: International journal of nanomedicine (2024): 633-650
Authors: Gu, Yu and Lozach, Pierre-Yves
Journal: Molecular microbiology (2024): 671-678
Authors: Thiele, Pia J and Mela-Lopez, Raquel and Blandin, Stéphanie A and Klug, Dennis
Journal: Malaria journal (2024): 114
Authors: Zhang, Wei and Xiang, Shuo and Long, Yanyang and Han, Yuxin and Jiang, Kang and Bian, Panpan and Weng, Qunhong
Journal: ACS applied materials & interfaces (2024): 342-352
Authors: Reja, Shahi Imam and Minoshima, Masafumi and Hori, Yuichiro and Kikuchi, Kazuya
Journal: Biosensors & bioelectronics (2024): 115862
Authors: Levin, Naamah and Hendler-Neumark, Adi and Kamber, Dotan and Bisker, Gili
Journal: Journal of colloid and interface science (2024): 650-666
Authors: Ghorai, Soumitra and Ghosh, Santu and Garai, Puja and Sen, Kaushik and Dash, Pratik Swarup and Jana, Nikhil R
Journal: ACS applied bio materials (2024): 443-451
Authors: He, Yating and Jiang, Kemei and Liu, Bojun and Meng, Hong-Min and Li, Zhaohui
Journal: Analytica chimica acta (2024): 342085