Amplite® Universal Fluorimetric MMP Activity Assay Kit *Red Fluorescence*
Example protocol
AT A GLANCE
Protocol summary
- Prepare appropriate controls or test samples (50 µL)
- Pre-incubate for 10 -15 minutes
- Add MMP RedTM Substrate working solution (50 µL)
- Skip incubation for kinetic reading or incubate 30 minutes - 1 hour for end point reading
- Monitor fluorescence intensity at Ex/Em = 540/590 nm (Cutoff = 570 nm)
Important notes
Thaw all the kit Components at room temperature before starting the experiment.
PREPARATION OF WORKING SOLUTION
1. APMA working solution (2mM, 2X):
Dilute 1 M APMA (Component B) with Assay Buffer (Component C) at 1:500 to make 2 mM, 2X APMA working solution . Note: APMA belongs to organic mercury. Handle with care! Dispose it according to local regulations.
2. MMP RedTM Substrate working solution:
Add 50 µL of MMP RedTM Substrate (Component A) to 5 mL of Assay Buffer (Component C) and mix well to make MMP RedTM Substrate working solution. Note: MMP RedTM Substrate working solution is enough for one 96-well plate (100 assays).
3. MMP dilution:
Dilulte MMPs to an appropriate concentration with Assay Buffer (Component C) if purified MMP is used. Note: Pro-MMP needs to be activated before use. Avoid vigorously vortexing the enzyme.
4. Inhibitors and compounds dilutions:
Make dilutions of known MMPs inhibitors and test compounds as desired if you are screening MMPs inhibitors.
For guidelines on cell sample preparation, please visit
https://www.aatbio.com/resources/guides/cell-sample-preparation.html
SAMPLE EXPERIMENTAL PROTOCOL
Table 1. Protocols for pro-MMP activation
| MMPs | Activated by Treating with |
| MMP-1 (collagenase) | 1 mM APMA (diluted component C) at 37 °C for 3 hr. |
| MMP-2 (gelatinase) | 1 mM APMA (diluted component C) at 37 °C for 1 hr. |
| MMP-3 (stromelysin) | 1 mM APMA (diluted component C) at 37 °C for 24 hr. |
| MMP-7 (matrilysin, PUMP-1) | 1 mM APMA (diluted component C) at 37 °C for 20 min-1 hr. |
| MMP-8 (neutrophil collagenase) | 1 mM APMA (diluted component C) at 37 °C for 1 hr. |
| MMP-9 (92 kDa gelatinase) | 1 mM APMA (diluted component C) at 37 °C for 2 hr. |
| MMP-10 (stromelysin 2) | 1 mM APMA (diluted component C) at 37 °C for 24 hr. |
| MMP-11 (stromelysin-3) | Already in active form. No APMA treatment is necessary. |
| MMP-12 (macrophage elastase) | 1 mM APMA (diluted component C) at 37 °C for 2 hr. |
| MMP-13 (collagenase-3) | 1 mM APMA (diluted component C) at 37 °C for 40 min. |
| MMP-14 | 1 mM APMA (diluted component C) at 37 °C for 2-3 hr. |
Table 2. Layout of the samples in a solid black 96-well microplate. SC=Substrate Control, IC=Inhibitor Control, VC=Vehicle Control, TC=Test Compound Control, TS=Test Samples.
| SC | SC | ... | ... |
| IC | IC | ... | ... |
| VC | VC | ||
| TC | TC | ||
| TS | TS | ||
| ... | ... | ||
Table 3. Reagent composition for each well. Some strongly fluorescent test compounds may result in false-positive results.
| Well | Volume | Reagent |
| SC | 50 µL | Assay Buffer |
| IC |
50 µL |
MMP dilution and known MMPs inhibitor |
| VC | 50 µL |
MMP dilution and vehicle used to deliver test compound |
| TC | 50 µL | Assay Buffer and test compound |
| TS | 50 µL |
MMP dilution with test compound |
- Prepare MMPs containing biological samples as desired.
- Incubate the MMP containing-samples or purified MMPs with equal volume of 2 mM APMA working solution (2X). Refer to Table 1 for incubation time. Activate MMP immediately before the experiment. Note: Keep enzyme-containing samples on ice. Avoid vigorously vortexing the enzyme. Prolonged storage of the activated enzyme will deactivate the enzyme. Note: For enzyme activation, it is preferably activated at higher protein concentration. After activation, you may further dilute the enzyme.
- Prepare Subtrate Control (SC), Inhibitor Control (IC), Vehicle Control (VC), Test Compound Control (TC) and Test Samples (TS) according to the layout provided in Table 2 and Table 3.
- Pre-incubate the plate at a desired temperature for the enzyme reaction (e.g. 25 °C or 37 °C) for 10-15 min if you are screening MMPs inhibitors.
- Add 50 µL/well (96-well plate) or 20 µL/well (384-well plate) of MMP Red™ Substrate working solution to the sample and control wells of the assay plate. Mix the reagents well.
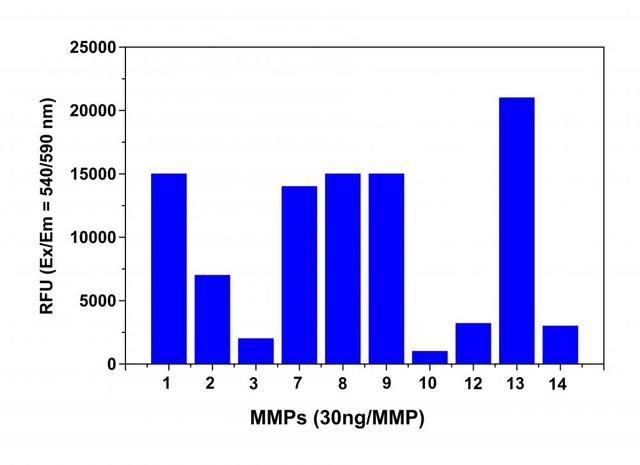
- Monitor the fluorescence intensity with a fluorescence plate reader at Ex/Em = 540/590 nm (Cutoff = 570 nm).
For kinetic reading: Immediately start measuring fluorescence intensity continuously and record data every 5 minutes for 30 minutes.
For end-point reading: Incubate the reaction at a desired temperature for 30 to 60 minutes, protected from light. Mix the reagents well, and then measure the fluorescence intensity.
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) |
| Amplite® Universal Fluorimetric MMP Activity Assay Kit *Green Fluorescence* | 494 | 515 |
Citations
Authors: She, Ziwei and Dong, Haosheng and Li, Yang and Chen, Ping and Zhou, Chunyan and Wang, Weiping and Jia, Zhuqing and Shi, Qiong
Journal: Experimental Cell Research (2024): 114288
Authors: Boddu, Vijay Kumar and Zamzow, Piet and Kramer, Mario Wolfgang and Merseburger, Axel S and Gorantla, Sivahari Prasad and Klinger, Matthias and Cramer, Lena and Sauer, Thorben and Gemoll, Timo and von Bubnoff, Nikolas and others,
Journal: Cell Communication and Signaling (2024): 1--14
Authors: Shieh, Jiunn-Min and Chang, Ting-Wei and Wang, Jing-He and Liang, Song-Ping and Kao, Pei-Lu and Chen, Liang-Yi and Yen, Chia-Jui and Chen, Yun-Ju and Chang, Wen-Chang and Chen, Ben-Kuen
Journal: The FASEB Journal (2023): e23206
Authors: Akentieva, Natalia and Sanina, Natalia and Gizatullin, Artur and Shkondina, Natalia and Andreeva, Anna and Shram, Stanislav and Aldoshin, Sergei
Journal: (2022)
Authors: Xiao, Ruyue and Yuan, Lan and He, Weijiang and Yang, Xiaoda
Journal: Metallomics (2018)