Cell Meter™ Fluorimetric Mitochondrial Superoxide Activity Assay Kit
Optimized for Microplate Reader
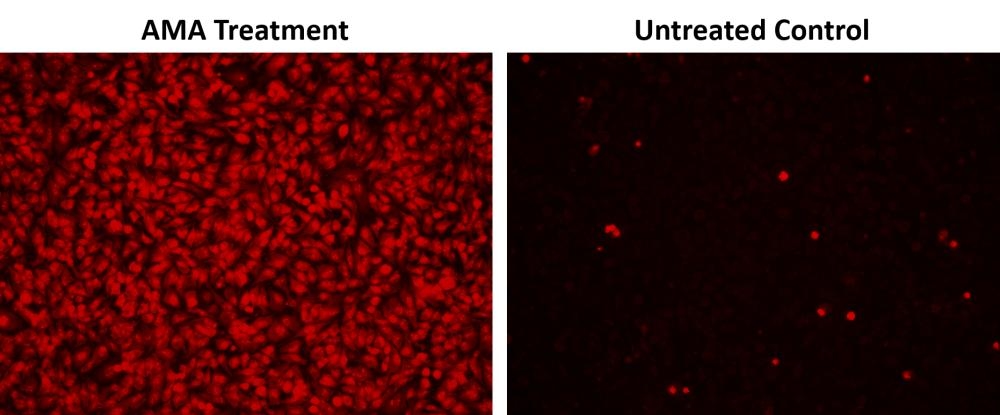
Mitochondria are major producers of cellular superoxide. The production of low to moderate levels of superoxide is critical for the proper regulation of many essential cellular processes including gene expression, signal transduction, and muscle adaptation to endurance exercise training. Uncontrolled mitochondrial superoxide production can trigger cellular oxidative damage that contributes to the pathogenesis of a wide variety of disorders including cancer, cardiovascular diseases, neurodegenerative diseases and aging. The detection of intracellular mitochondrial superoxide is of central importance to understanding proper cellular redox regulation and the impact of its dysregulation on various pathologies. Cell Meter™ Fluorimetric Mitochondrial Superoxide Activity Assay Kit uses our unique Superoxide Indicator to quantify superoxide level in live cells. MitoROS™ 580 is live-cell permeant and can rapidly and selectively target superoxide in mitochondria. It generates red fluorescence when it reacts with superoxide. The Cell Meter™ Fluorimetric Intracellular Superoxide Detection Kit provides a sensitive, one-step fluorimetric assay to detect mitochondrial superoxide in live cells with one hour incubation. This kit can be used for fluorescence microplate readers and fluorescence microscopy applications.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22971 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 500 |
| Emission (nm) | 582 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026

