Cell Meter™ JC-10 Mitochondrion Membrane Potential Assay Kit
Optimized for Flow Cytometry Assays
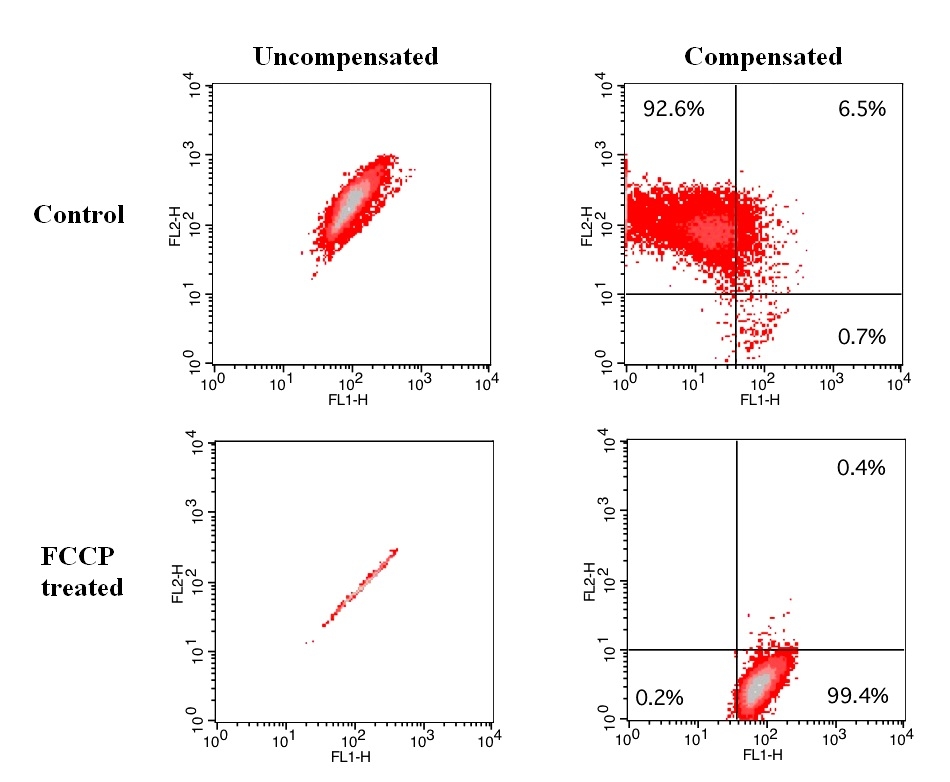
Although JC-1 is widely used in many labs, its poor water solubility causes extraordinary inconvenience. Even at 1 µM concentration, JC-1 tends to precipitate in aqueous buffer. JC-10 is developed to be a superior alternative to JC-1 where high dye concentration is desired. Compared to JC-1, JC-10 has much better water solubility. JC-10 is capable of entering selectively into mitochondria, and changes reversibly its color from green to orange as membrane potentials increase. This property is due to the reversible formation of JC-10 aggregates upon membrane polarization that causes shifts in emitted light from 520 nm (i.e., emission of JC-10 monomeric form) to 570 nm (i.e., emission of J-aggregate). When excited at 490 nm, the color of JC-10 changes reversibly from green to greenish orange as the mitochondrial membrane becomes more polarized. Both colors can be detected using the filters commonly mounted in all flow cytometers, so that green emission can be analyzed in fluorescence channel 1 (FL1) and greenish orange emission in channel 2 (FL2). Besides its potential use in flow cytometry, it can also be used in fluorescence imaging and fluorescence microplate platform. This kit provides all the essential components with an optimized assay method for the detection of apoptosis in cells with the loss of mitochondrial membrane potential. This fluorometric assay is based on the detection of the mitochondrial membrane potential changes in cells by the cationic, lipophilic JC-10 dye. In normal cells, JC-10 concentrates in the mitochondrial matrix where it forms red fluorescent aggregates. However, in apoptotic and necrotic cells, JC-10 exists in monomeric form and stains cells in green fluorescence. The kit is optimized for screening of apoptosis activators and inhibitors by flow cytometry. We also offer a convenient 96-well and 384-well fluorescence microtiter-plate format kit (cat#22800) for high through put screening.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22801 | 100 Tests | Price |
Spectral properties
| Excitation (nm) | 508 |
| Emission (nm) | 524 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm, 575/26 nm filter |
| Instrument specification(s) | FITC, PE channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 30, 2026

