Cyanine 5.5 monosuccinimidyl ester
equivalent to Cy5.5® NHS ester
A variety of cyanine 5.5 (Cy5.5®) dyes has been used to label biological molecules for fluorescence imaging and other fluorescence-based biochemical analysis. They are widely used for labeling peptides, proteins and oligos etc. Cy5.5® dyes are one type of the most common red fluorophores. Cy5.5® NHS ester readily reacts with amino groups. AAT Bioquest offers Cy dye NHS esters in the form of triethylammonium salts that are more soluble in DMSO and DMF than the corresponding potassium salts that are offered by some other vendors. The Cy dye triethylammonium salts have the same reactivity and give the conjugates identical to the the Cy dye potassium salts. Cy5.5® is the trademark of GE Healthcare.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 174 | 1 mg | Price |
Physical properties
| Molecular weight | 1317.70 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.05 |
| Correction factor (280 nm) | 0.101 |
| Correction factor (482 nm) | 0.0017 |
| Correction factor (565 nm) | 0.047 |
| Correction factor (650 nm) | 0.454 |
| Extinction coefficient (cm -1 M -1) | 250000 |
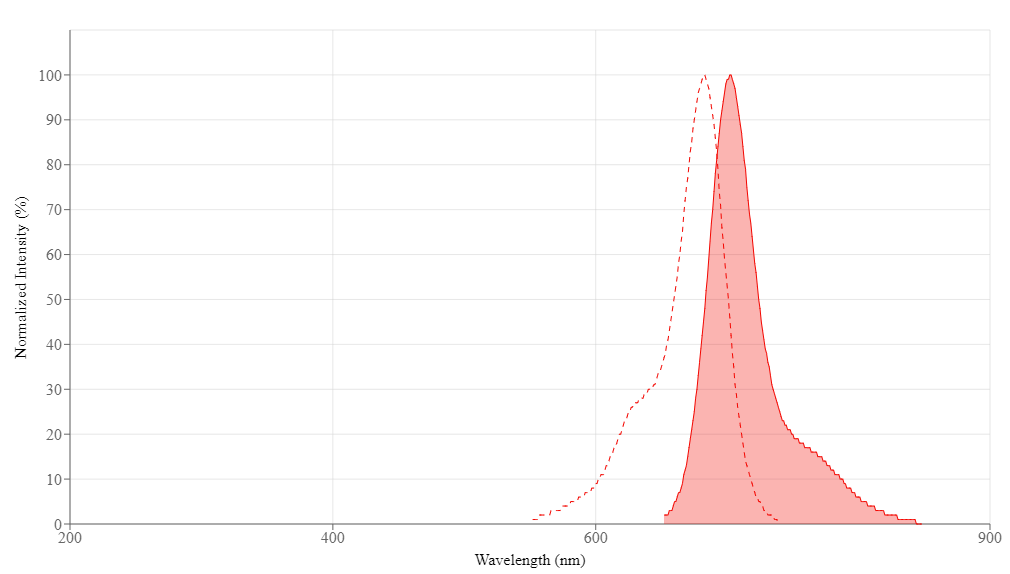
| Excitation (nm) | 683 |
| Emission (nm) | 703 |
| Quantum yield | 0.27 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026

