mFluor™ Red 780 Maleimide
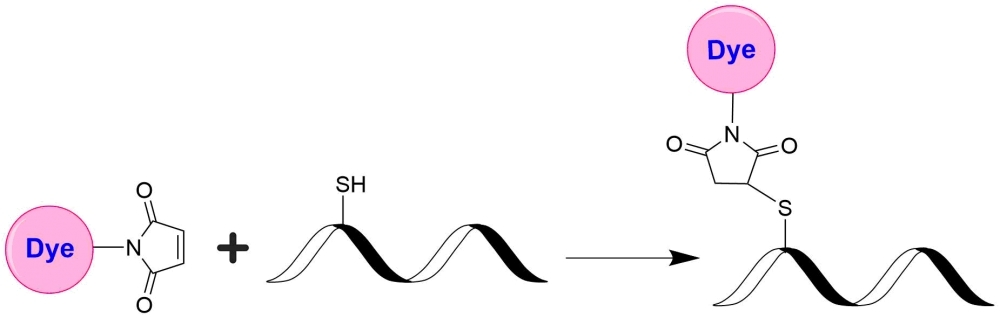
mFluor™ Red 780 dyes are an excellent alternative to APC-Alexa Fluor® 750 tandems since they have the spectral properties equivalent to those of APC-Alexa Fluor® 750 conjugates. mFluor™ Red 780 dyes are water-soluble, and the protein conjugates prepared with mFluor™ Red 780 dyes are well excited at 633 nm or 647 nm to give red fluorescence (compatible with Cy7® filter). mFluor™ Red 780 dyes and conjugates are excellent red laser reagents for flow cytometry research. Compared to APC-Alexa Fluor® 750 tandems, mFluor™ Red 780 dyes are much more photostable, making them readily available for fluorescence imaging applications while it is very difficult to use the APC-Alexa Fluor® 750 conjugates for fluorescence imaging applications due to the rapid photobleaching of APC-Alexa Fluor® 750 tandems. mFluor™ Red 780 maleimide is stable and highly reacts with thiol-containing biomolecules such as reduced antibodies.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1192 | 1 mg | Price |
Physical properties
| Molecular weight | 1168.28 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 630 |
| Correction factor (260 nm) | 0.101 |
| Correction factor (280 nm) | 0.116 |
| Extinction coefficient (cm -1 M -1) | 90000 1 |
| Excitation (nm) | 629 |
| Emission (nm) | 767 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on December 14, 2025

