Portelite™ Fluorimetric High Sensitivity DNA Quantitation Kit
Optimized for CytoCite™ and Qubit™ Fluorometers
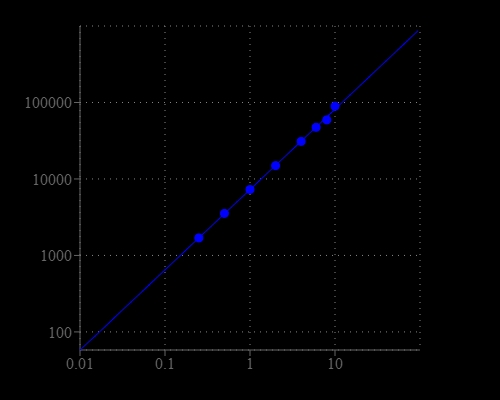
DNA Quantitation is a very important task in DNA sample preparations for various genomic analyses. This Portelite™ dsDNA Quantitation Kit provides a rapid method to quantify dsDNA with Helixyte™ Green probe using a hand-held fluorometer. It is optimized for Cytocite™ and Qubit™ fluorometers. Portelite™ dsDNA Quantitation assay is linear over five orders of magnitude. The assay is highly selective for double-stranded DNA (dsDNA) over RNA and is designed to be accurate for initial sample concentrations from 1.25 pg/uL to 100 ng/uL. Helixyte™ Green exhibits large fluorescence enhancement upon binding to dsDNA, and it is a few magnitudes more sensitive than UV absorbance readings.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 17660 | 100 Tests | Price | |
| 17661 | 500 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H340 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R68 |
| UNSPSC | 41116134 |
Instrument settings
| Qubit Fluorometer | |
| Excitation | 480 nm |
| Emission | 520 nm |
| Instrument specification(s) | 0.2 mL, thin-wall PCR tube |
| CytoCite Fluorometer | |
| Excitation | 480 nm |
| Emission | 520 nm |
| Instrument specification(s) | 0.2 mL, thin-wall PCR tube |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
