ReadiUse™ Staurosporine
1 mM DMSO stock solution
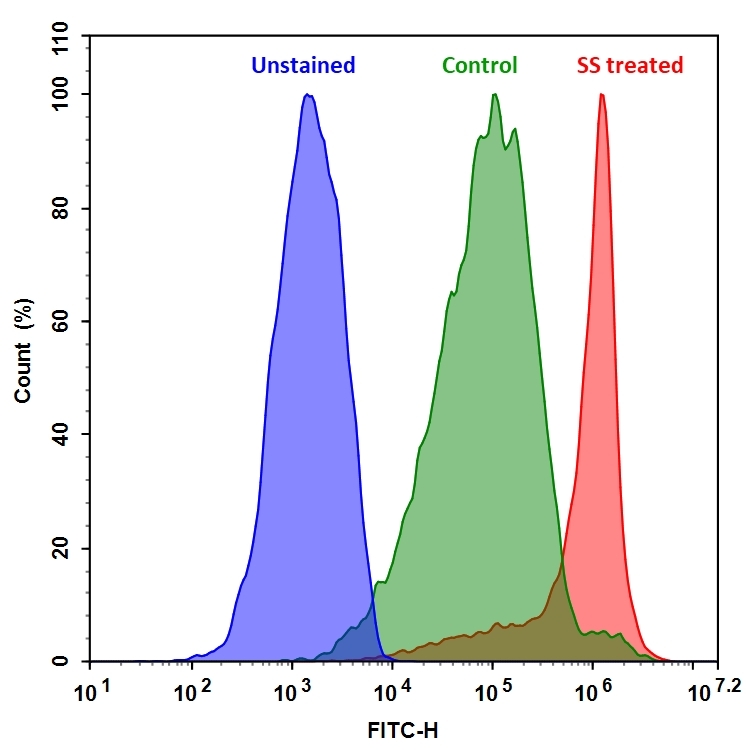
Staurosporine is widely used as a positive control for inducing apoptosis. It has been proven that Staurosporine induces apoptosis in human malignant glioma cell lines, and arrests normal cells at the G1 checkpoint. Staurosporine is a potent, cell-permeable, reversible, ATP-competitive and broad spectrum inhibitor of protein kinases, e.g., CaM kinase (IC50 = 20 nM), myosin light chain kinase (IC50 = 1.3 nM), protein kinase A (IC50 = 7 nM), protein kinase C (IC50 = 0.7 nM), and protein kinase G (IC50 = 8.5 nM). Staurosporine also inhibits platelet aggregation induced by collagen or ADP but has no effect on thrombin-induced platelet aggregation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 80050 | 100 uL | Price |
Physical properties
| Molecular weight | 466.54 |
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| CAS | 62996-74 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 18, 2026
