Screen Quest™ Membrane Potential Assay Kit
Orange Fluorescence
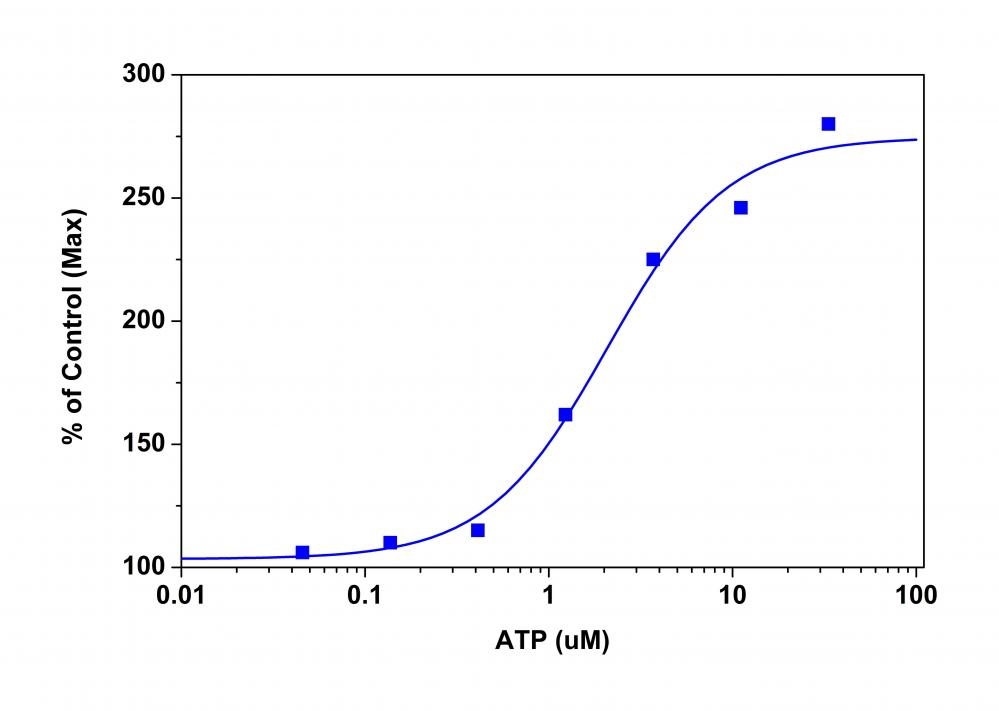
Membrane potential is the difference in voltage between the interior and exterior of a cell. The membrane potential allows a cell to function as a battery, providing power to operate a variety of "molecular devices" embedded in the membrane. In electrically excitable cells such as neurons, membrane potential is used for transmitting signals between different parts of a cell. Opening or closing of ion channels at one point in the membrane produces a local change in the membrane potential, which causes electric current to flow rapidly to other points in the membrane. Ion channels have been identified as important drug discovery targets. Our Screen Quest™ Membrane Potential Assay Kit is a homogeneous assay with fast read time. It uses our proprietary long wavelength membrane potential indicator to detect the membrane potential change that is caused by the opening and closing of the ion channels. The red fluorescence of the membrane potential indicator used in the kit has enhanced fluorescence upon entering cells and minimizes the interferences resulted from the screening compounds and/or cellular autofluorescence.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 35999 | 1 plate | Price | |
| 36000 | 10 plates | Price | |
| 36001 | 100 plates | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 530 nm |
| Emission | 570 nm |
| Cutoff | 550 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
| Other instruments | FDSS, FLIPR, FlexStation, NOVOStar |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 8, 2026
