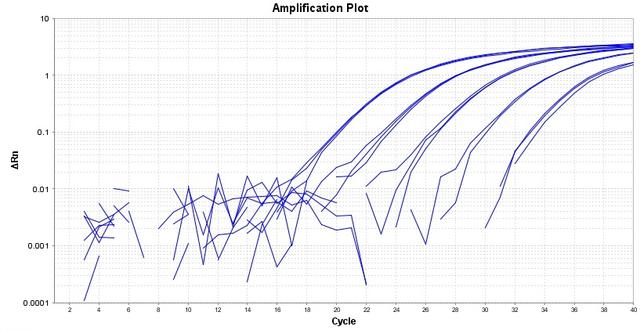
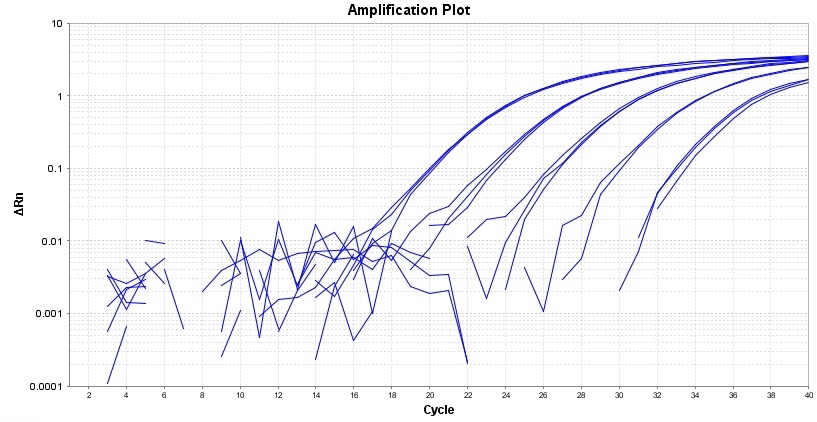
TAQuest™ FAST qPCR Master Mix for TaqMan Probes *Low ROX*
TAQuest™ FAST qPCR Master Mix for TaqMan Probes is a ready-to-use 2X solution optimized for qPCR and 2-step RT-qPCR well suited to use for TaqMan gene expression assays. The master mix is compatible with FAST conditions, thus delivers results within 50 minutes for 40 cycles of PCR in a 20 uL reaction volume. The master mix provides all of the essential components including our proprietary TAQuest™ FAST Hot Start Taq DNA Polymerase enzyme and dNTPs in an optimized PCR buffer, except the template, primers and probes. TAQuest™ FAST qPCR Master Mix for TaqMan Probes has been designed to be used for duplex reactions using internal positive controls with superior performance. The master mix ensures PCR specificity and sensitivity with all sample types such as genomic, plasmid, viral and cDNA templates. This master mix contains a low amount of ROX reference dye.
Example protocol
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol can be used as a guideline.
Note Thaw the TAQuest™ FAST qPCR Master Mix for TaqMan Probes *Low ROX* at room temperature. Vortex qPCR Master Mix thoroughly before use.
Table 2.Thermal cycling parameters
Note Thaw the TAQuest™ FAST qPCR Master Mix for TaqMan Probes *Low ROX* at room temperature. Vortex qPCR Master Mix thoroughly before use.
- Prepare one of the following reaction mixes as indicated in Table 1.
- Carefully mix the reagents with a gentle vortex followed by a brief centrifuge.
- Set up the plate in the qPCR instrument and run as indicated in Table 2.
| Components | Volume (25 µL/reaction) | Volume (50 µL/reaction) | Final Conc. |
| TAQuest™ FAST qPCR Master Mix for TaqMan Probes *Low ROX* | 12.5 µL | 25 µL | 1X |
| Upstream primer, 10 µM | 0.25-2.5 µL | 0.5-5.0 µL | 0.1-1.0 µM |
| Downstream primer, 10 µM | 0.25-2.5 µL | 0.5-5.0 µL | 0.1-1.0 µM |
| TaqMan Probes, 10 µM | 0.25-0.625 µL | 0.5-1.25 µL | 100-250 nM |
| DNA template | 1-5 µL | 1-5 µL | Optimized conc. |
| Nuclease-Free Water to | 25 µL | 50 µL |
| Parameter | Polymerase Activation | PCR (30-40 cycles) | |
| Hold | Denature | Anneal/Extend | |
| Temperature | 95 °C | 95 °C | 60 °C |
| Time (m:ss) | 0:10 | 0:20 | 0:30 |
References
View all 50 references: Citation Explorer
Evaluation of the efficiency of TaqMan duplex real-time PCR assay for non-invasive pre-natal assessment of foetal sex in equine.
Authors: Kadivar, Ali and Rashidzadeh, Habiballah and Davoodian, Najmeh and Nazari, Hasan and Dehghani Tafti, Rohallah and Heidari Khoei, Heidar and Seidi Samani, Hasan and Modaresi, Jahangir and Ahmadi, Ebrahim
Journal: Reproduction in domestic animals = Zuchthygiene (2021): 287-291
Authors: Kadivar, Ali and Rashidzadeh, Habiballah and Davoodian, Najmeh and Nazari, Hasan and Dehghani Tafti, Rohallah and Heidari Khoei, Heidar and Seidi Samani, Hasan and Modaresi, Jahangir and Ahmadi, Ebrahim
Journal: Reproduction in domestic animals = Zuchthygiene (2021): 287-291
Fast and Sensitive Real-Time PCR Detection of Major Antiviral-Drug Resistance Mutations in Chronic Hepatitis B Patients by Use of a Predesigned Panel of Locked-Nucleic-Acid TaqMan Probes.
Authors: Chu, Son V and Vu, Son T and Nguyen, Hang M and Le, Ngan T and Truong, Phuong T and Vu, Van T T and Phung, Thuy T B and Nguyen, Anh T V
Journal: Journal of clinical microbiology (2021): e0093621
Authors: Chu, Son V and Vu, Son T and Nguyen, Hang M and Le, Ngan T and Truong, Phuong T and Vu, Van T T and Phung, Thuy T B and Nguyen, Anh T V
Journal: Journal of clinical microbiology (2021): e0093621
Multiplex TaqMan Real-Time PCR Assay for Sensitive Detection of Two Weevil Species (Coleoptera: Curculionidae).
Authors: Aguirre, Carlos and Sánchez, Evelyn and Olivares, Natalia and Hinrichsen, Patricio
Journal: Journal of economic entomology (2021): 90-99
Authors: Aguirre, Carlos and Sánchez, Evelyn and Olivares, Natalia and Hinrichsen, Patricio
Journal: Journal of economic entomology (2021): 90-99
A development strategy to fast establish the Taqman qPCR based method to detect SNP mutations.
Authors: Jiang, Xiaohui and Xiang, Junbei and Wang, Ruifeng and Wan, Qian
Journal: Human cell (2020): 1331-1333
Authors: Jiang, Xiaohui and Xiang, Junbei and Wang, Ruifeng and Wan, Qian
Journal: Human cell (2020): 1331-1333
One-step multiplex TaqMan probe-based method for real-time PCR detection of four canine diarrhea viruses.
Authors: Wang, Ruyi and Zhang, Wenyan and Ye, Rui and Pan, Zhongzhou and Li, Gairu and Su, Shuo
Journal: Molecular and cellular probes (2020): 101618
Authors: Wang, Ruyi and Zhang, Wenyan and Ye, Rui and Pan, Zhongzhou and Li, Gairu and Su, Shuo
Journal: Molecular and cellular probes (2020): 101618
Page updated on August 12, 2025