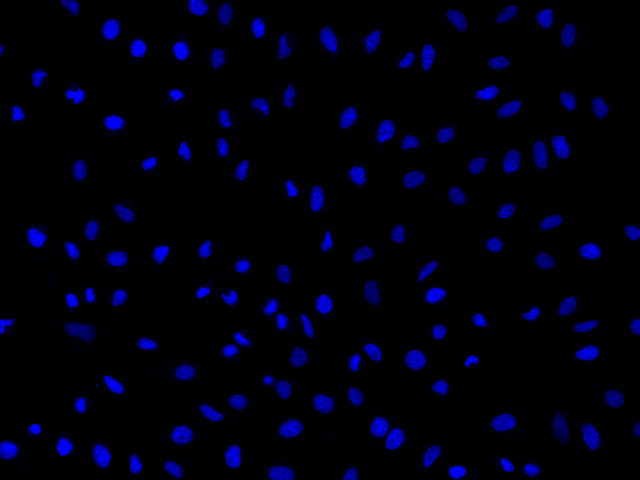
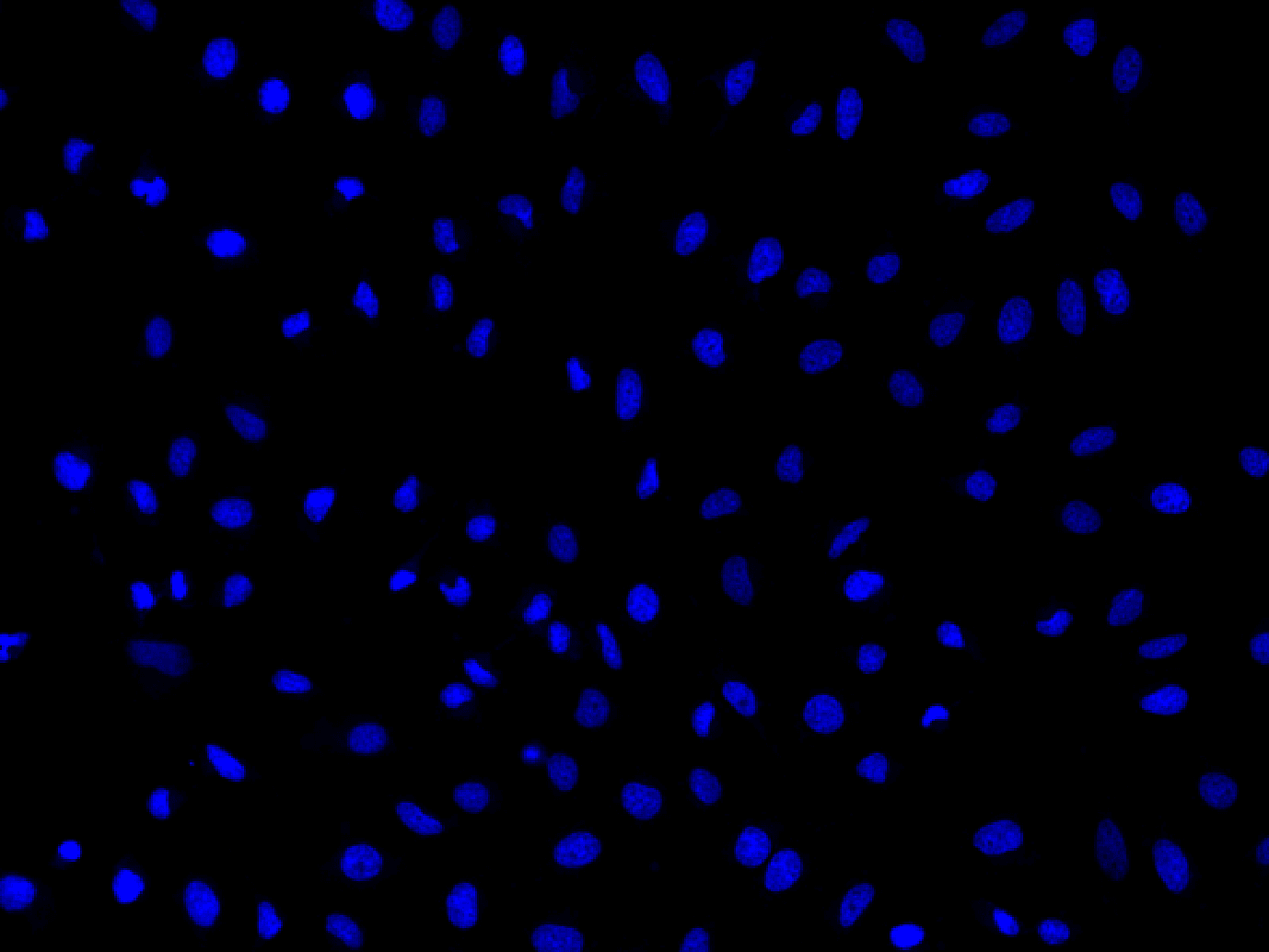
Nuclear Blue™ LCS1
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | 822.69 |
| Solvent | DMSO |
| Excitation (nm) | 353 |
| Emission (nm) | 456 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 41116134 |
| Overview |
Molecular weight 822.69 | Excitation (nm) 353 | Emission (nm) 456 |
Platform
Fluorescence microscope
| Excitation | DAPI Filter |
| Emission | DAPI Filter |
| Recommended plate | Black wall/clear bottom |
Example protocol
AT A GLANCE
Ex/Em = 353/456 nm (bound to DNA)
SAMPLE EXPERIMENTAL PROTOCOL
Caution: The following protocol can be adapted for most cell types. Growth medium, cell density, the presence of other cell types, and factors may influence staining. Residual detergent on glassware may also affect the staining of many organisms and cause brightly stained material to appear in solutions with or without cells present.
Add Nuclear Blue™ LCS1 (2 to10 µM) directly into the live cell culture medium (either suspension or adherent) and incubate the cells for 15 to 60 minutes.
Note: In initial experiments, it is advisable to test a wide range of dye concentrations in order to determine the optimal concentration that yields the desired result.
Wash the cells twice with Hanks and 20 mM HEPES buffer (HBSS) or a buffer of your choice. Then fill the wells with fresh HBSS or growth medium.
Observe the cells using a fluorescence microscope, fluorescence microplate reader, or flow cytometer equipped with the desired filter set.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 121.552 µL | 607.762 µL | 1.216 mL | 6.078 mL | 12.155 mL |
| 5 mM | 24.31 µL | 121.552 µL | 243.105 µL | 1.216 mL | 2.431 mL |
| 10 mM | 12.155 µL | 60.776 µL | 121.552 µL | 607.762 µL | 1.216 mL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Product Family
| Name | Excitation (nm) | Emission (nm) |
| Nuclear Blue™ DCS1 *5 mM DMSO Solution* | 348 | 469 |
| Nuclear Blue™ DCS2 *Equivalent to SYTOX™ Blue Dead Cell Stain* | 445 | 470 |
| Nuclear Green™ LCS1 *5 mM DMSO Solution* | 503 | 527 |
| Nuclear Orange™ LCS1 *5 mM DMSO Solution* | 514 | 556 |
| Nuclear Red™ LCS1 *5 mM DMSO Solution* | 622 | 645 |
| Nuclear Violet™ LCS1 *5 mM DMSO Solution* | 401 | 460 |
Images
References
Authors: Fuchs, Heiko and Jahn, Kirsten and Hu, Xiaonan and Meister, Roland and Binter, Maximilian and Framme, Carsten
Journal: Advanced healthcare materials (2023): e2300230
Authors: Arvidsson, Malou and Rashed, Salma Kazemi and Aits, Sonja
Journal: Data in brief (2023): 108769
Authors: Merolli, Antonio and Bektas, Cemile
Journal: Journal of anatomy (2022): 998-1001
Authors: Wang, Qin and Lü, Li-Bing and Tao, Zhu and Sun, Tao and Tang, Qing and Huang, Ying
Journal: Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy (2021): 119656
Authors: Swain, Brendan M and Guo, Dawei and Singh, Himansha and Rawlins, Philip B and McAlister, Mark and van Veen, Hendrik W
Journal: Scientific reports (2020): 20026
Authors: Eddaoudi, Ayad and Canning, Stephanie Louise and Kato, Itaru
Journal: Methods in molecular biology (Clifton, N.J.) (2018): 49-57
Authors: Kawski, A and Gryczyński, Z and Gryczyński, I and Lakowicz, J R and Piszczek, G
Journal: Zeitschrift fur Naturforschung. A, Journal of physical sciences (2016): 1037-1041
Authors: Schendzielorz, P and Froelich, K and Rak, K and Gehrke, T and Scherzad, A and Hagen, R and Radeloff, A
Journal: Stem cells international (2016): 6549347
Authors: DeSouza, Ngoc and Zhou, Megan and Shan, Yi
Journal: Methods in molecular biology (Clifton, N.J.) (2016): 47-57
Authors: Crowley, Lisa C and Marfell, Brooke J and Waterhouse, Nigel J
Journal: Cold Spring Harbor protocols (2016)
Application notes
Fluorescent Oligonucleotide Labeling Reagents
Monitoring of Mitochondrial Membrane Potential Changes in Live Cells Using JC-10
Selective Analysis of RNA in Live and Fixed Cells with StrandBrite RNA Green
Cell Loading Protocol For Fluorescent pH Indicator, BCECF-AM
FAQ
What dye works best for staining and tracking lysosomes in live cells for several hours?
How can I lyse my cells without lysing the nuclear membrane?
Do you have any dual-fluorescence nucleic acid stains that interact with both DNA and RNA?
Do you have any fixable mitochondria staining assay kits?