Cell Meter™ Fluorimetric Live Cell Cycle Assay Kit
Red Fluorescence Optimized for Flow Cytometry
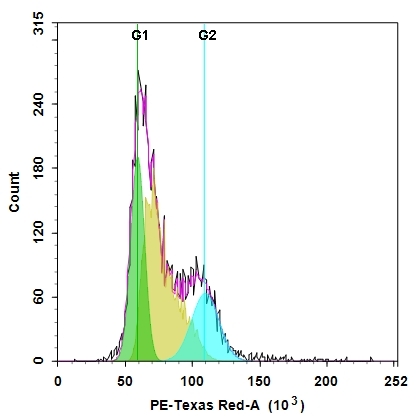
Cell cycles have four sequential phases: G0/G1, S, G2, and M. During a cell's passage through cell cycle, its DNA is duplicated in S (synthesis) phase and distributed equally between two daughter cells in M (mitosis) phase. These two phases are separated by two gap phases: G0/G1 and G2. The two gap phases provide time for cells to grow and double the mass of their proteins and organelles. They are also used by cells to monitor internal and external conditions before proceeding with the next phase of cell cycle. The cell's passage through cell cycles is controlled by a host of different regulatory proteins. This assay kit is designed to monitor cell cycle progression and proliferation by using our proprietary Nuclear Red™ CCS2 in live cells. The percentage of cells in a given sample that are in G0/G1, S and G2/M phases, as well as the cells in the sub-G1 phase prior to apoptosis can be determined by flow cytometry. Cells stained with Nuclear Red™ CCS2 can be monitored with a flow cytometer (PE-Texas Red channel).

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22860 | 100 tests | Price |
Spectral properties
| Excitation (nm) | 542 |
| Emission (nm) | 580 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 610/20 nm filter |
| Instrument specification(s) | PE-Texas Red channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 5, 2026

