CytoTell® UltraGreen
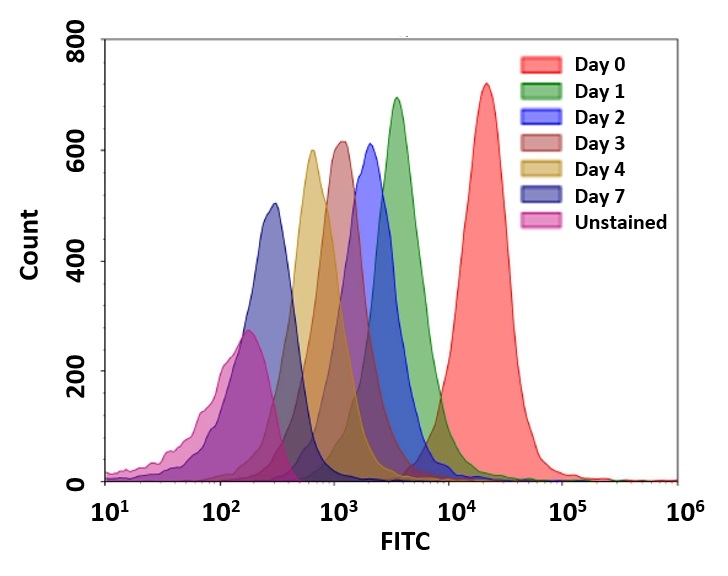
Flow cytometry combined with fluorescence staining is a powerful tool to analyze heterogeneous cell populations. Among all the existing fluorescent dyes CFSE is the preferred cell proliferation indicator that is widely used for live cell analysis. However, there are a few severe problems associated with the use of CFSE for monitoring cell proliferation. 1). CFSE is highly toxic to cells. CFSE indiscriminately reacts with all amino groups, thus changes many critical intracellular protein functions (such as cell membrane GPCRs); 2). CFSE has slow response and is inconvenient to use. The CFSE fluorescence intensity of the 2nd generation cells is decreased more than 10 fold from the 1st generation. You would have to wait for another generation to start the cell proliferation analysis. 3). Medium removal is required. You would have to remove medium for cell analysis with a flow cytometer since CFSE reacts with medium components. CytoTell® Green has been developed to eliminate these CFSE limitations. Based on our customers' feedbacks on our CytoTell® Green, CytoTell® UltraGreen is our newest improvement. It has distinct advantages. 1). CytoTell® UltraGreen is well retained in cells; 2). CytoTell® UltraGreen exhibits much faster response and is more convenient to use than CFSE. The fluorescence intensity gap between 1st and 2nd generation is significantly minimized. As cells divide, CytoTell® UltraGreen is distributed equally between daughter cells that can be measured as successive halving of the fluorescence intensity of the dye; 3). CytoTell® UltraGreen is more sensitive than CFSE. Up to 9 generations may be visualized; 4). CytoTell® UltraGreen is much more stable than CFSE. CytoTell® UltraGreen stock solution can be stored at room temperature for a few days. CytoTell® UltraGreen can also be used for long term tracking of labeled cells. Analysis using two-parameter plots may provide better resolution of each generation, especially between undivided cells and the first generation. CytoTell® UltraGreen has a peak excitation of 492 nm and can be excited by the blue (488 nm) laser line, making it compatible with FITC filter set.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22240 | 500 Tests | Price | |
| 22241 | 2x500 Tests | Price |
Physical properties
| Molecular weight | ~500 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 492 |
| Emission (nm) | 514 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 31, 2026

