MycoLight™ Ratiometric Bacterial Membrane Potential Kit
Red/Green Fluorescence
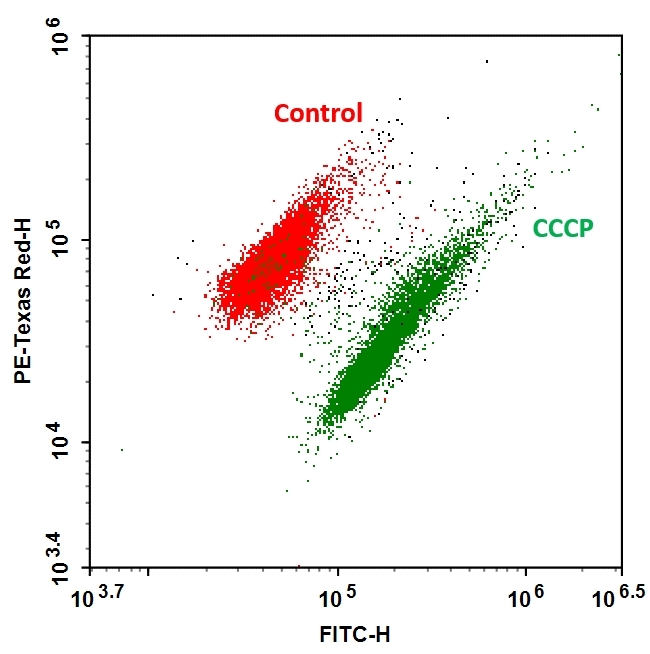
AAT Bioquest's MycoLight™ Ratiometric Bacterial Membrane Potential Kit uses a fluorescent sensor that exhibits green fluorescence in both gram-positive and negative bacterial cells in low concentration, but the fluorescence shifts toward red emission at higher cytosolic concentrations due to the dye molecule aggregation caused by larger membrane potentials. The magnitude of membrane potential varies with different bacterial species. For many gram-positive species, the red/green ratio tend to vary with the intensity of the proton gradient while in many gram-negative bacteria the response of the dye does not appear to be proportional to proton gradient intensity. This kit is designed to assay bacterial membrane potentials when the bacterial concentrations are in the range of 105 “ 107 organisms per mL. Stained cells can be monitored fluorimetrically at 510-530 nm (FITC filter) and 600-660 nm (Texas red filter) with excitation at 488 nm, the most common excitation light source.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22401 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 483 |
| Emission (nm) | 501 |
Storage, safety and handling
| H-phrase | H301, H311, H331 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R23, R24, R25 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm, 610/20 nm filter |
| Instrument specification(s) | FITC, PE-Texas Red channel |
| Fluorescence microscope | |
| Excitation | 510/600 nm |
| Emission | 530/660 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | FITC/Texas Red filter sets |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 8, 2026

