Portelite™ Fluorimetric RNA Quantification Kit
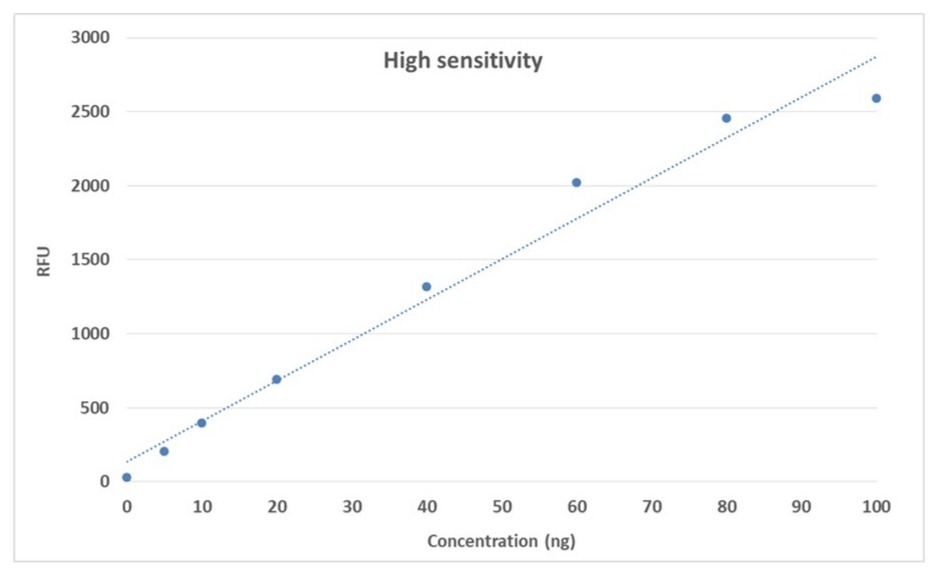
5-100 ng High Sensitivity
Portelite™ Fluorimetric RNA Quantification Assay Kit provides accurate RNA measurement at low concentrations for sensitive applications.
- Ultra-sensitive detection: Accurately quantifies RNA in the 5–100 ng range with high signal-to-background ratio
- High specificity: Selectively measures RNA with minimal interference from DNA, proteins, or free nucleotides
- Applications: Suitable for RT-qPCR, RNA-seq, cDNA synthesis, and other RNA-based studies requiring low input amounts
- Comparable alternative: Outperforms traditional absorbance-based RNA quantification kits by enabling accurate detection at low concentrations with reduced interference

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 17695 | 100 Tests | Price |
Physical properties
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Instrument settings
| Qubit Fluorometer | |
| Excitation | 635 nm |
| Emission | 665-720 nm |
| Instrument specification(s) | 0.2 mL PCR vial |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 27, 2026
