RatioWorks™ PH165
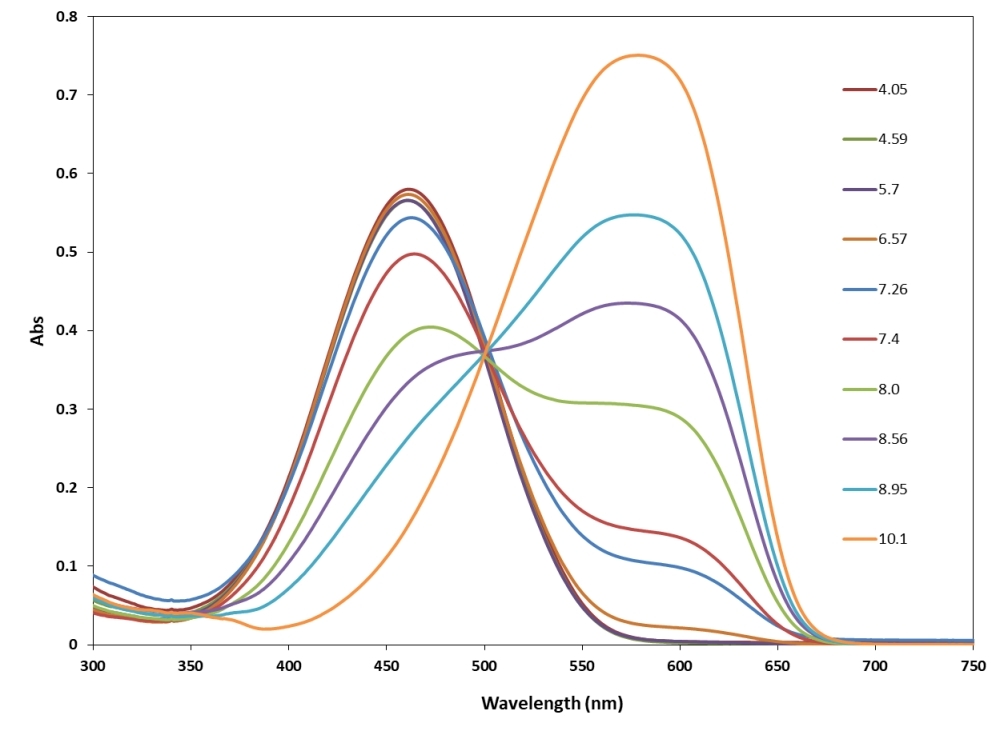
RatioWorks™ PH165 is a novel fluorescent probe designed for pH measurement in solution, featuring pH-dependent fluorescence spectra and unique dual excitation and dual emission properties. At low pH, RatioWorks™ PH165 is weakly fluorescent in the far-red spectrum region and highly fluorescent in the red spectrum region. As pH increases, it shows higher fluorescence in the far-red spectrum and lower fluorescence in the red spectrum. This probe quantifies pH in the 4 to 9 range, utilizing dual excitation/emission at 497/594 nm and 578/654 nm, respectively, and has a pKa of ~7.1. The red fluorescence spectrum of RatioWorks™ PH165 makes it suitable for multiplexing tests with green fluorophores like GFP. RatioWorks™ PH165 AM is cell-permeable and compatible with platforms such as fluorescence microscopy and microplate readers.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21213 | 10x50 ug | Price |
Physical properties
| Molecular weight | 517.54 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 463 |
| Emission (nm) | 641 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on December 19, 2025

