Screen Quest™ Fluorimetric ELISA cAMP Assay Kit
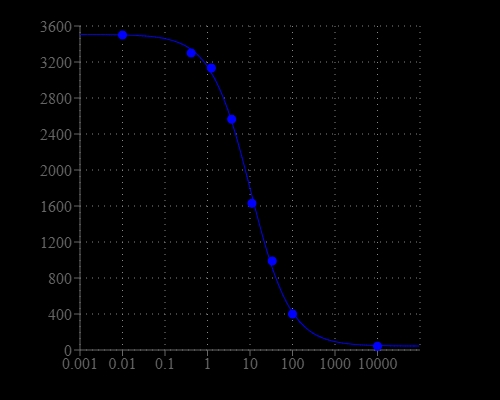
Screen Quest™ Fluorescence ELISA cAMP Assay Kit provides an optimized assay method for monitoring the activation of adenylyl cyclase in G-protein coupled receptor systems. The assay is based on the competition for a fixed number of antibody binding sites between HRP-labeled cAMP and non-labeled cAMP. HRP-cAMP is displaced from the HRP-cAMP/anti-cAMP antibody complex by unlabeled free cAMP. In the absence of cAMP, HRP-cAMP conjugate is bound to anti-cAMP antibody exclusively. However, the unlabeled free cAMP in the test sample competes for anti-cAMP antibody with the HRP-cAMP antibody conjugate, therefore inhibits the binding of HRP-cAMP to anti-cAMP antibody. Our Screen Quest™ Fluorometric cAMP Assay Kit provides a sensitive method for detecting adenylate cyclase activity. Compared to other commercial ELISA cAMP assay kits, this cAMP assay kit only requires a single wash step to remove unbound material prior to the development step. It also eliminates the tedious acetylation step. The kit uses Amplite® Red as a fluorogenic HRP substrate to quantify the HRP activity. The fluorescent product formed is proportional to the activity of HRP-cAMP conjugate.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36373 | 1 plate | Price | |
| 36374 | 10 plates | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black (Component H) |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 24, 2026

