Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit
Red Fluorescence
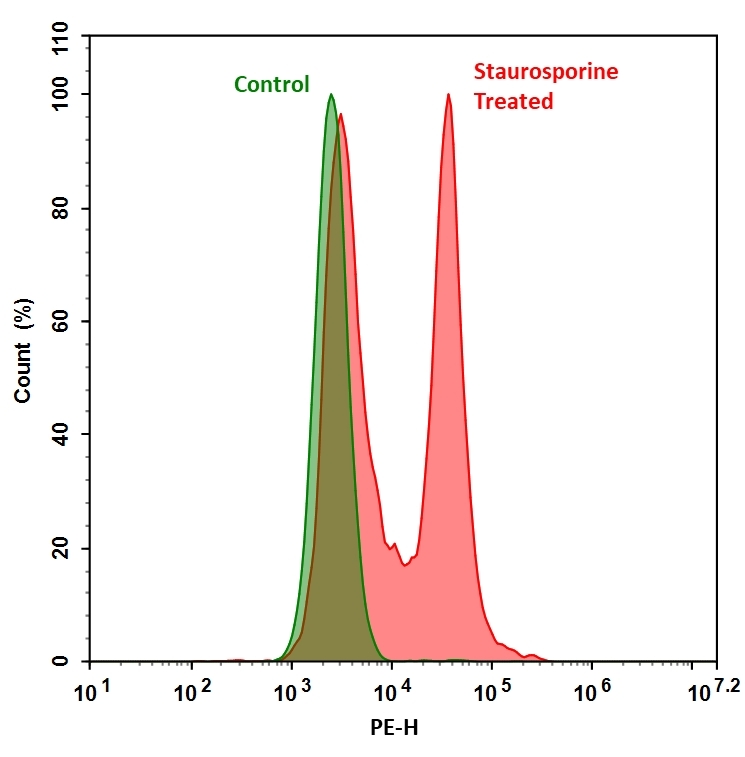
Our Cell Meter™ live cell caspases activity assay kits are based on fluorescent FMK inhibitors of caspases. These inhibitors are cell permeable and non-cytotoxic. Once inside the cell, the caspase inhibitors bind covalently to the active caspases. The activation of caspase 3/7 is important for the initiation of apoptosis. It has been proven that caspase 3/7 has substrate selectivity for the peptide sequence Asp-Glu-Val-Asp (DEVD). This kit uses TF3-DEVD-FMK as a fluorescent indicator for caspase 3/7 activity. TF3-DEVD-FMK irreversibly binds to activated caspase 3/7 in apoptotic cells. Once bound to caspase 3/7, the fluorescent reagent is retained inside the cell. The binding event inhibits caspase 3/7 but will not stop apoptosis from proceeding. There are a variety of parameters that can be used for monitoring cell apoptosis. This Cell Meter™ Live Cell Caspase 3/7 Activity Assay Kit is designed to detect cell apoptosis by measuring caspase 3/7 activation in live cells. It is used for the quantification of activated caspase 3/7 activities in apoptotic cells, or for screening caspase 3/7 inhibitors. TF3-DEVD-FMK, the red label reagent, allows for direct detection of activated caspase 3/7 in apoptotic cells by fluorescence microscopy, flow cytometer, or fluorescent microplate reader. The kit provides all the essential components with an optimized assay protocol.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20101 | 25 Tests | Price |
Spectral properties
| Correction factor (280 nm) | 0.179 |
| Extinction coefficient (cm -1 M -1) | 75000 1 |
| Excitation (nm) | 553 |
| Emission (nm) | 578 |
| Quantum yield | 0.1 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 550 nm |
| Emission | 595 nm |
| Instrument specification(s) | FL1 Channel |
| Fluorescence microscope | |
| Excitation | TRITC channel |
| Emission | TRITC channel |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | FITC channel for Nuclear Green™ DCS1 staining, DAPI channel for Hoechst staining |
| Fluorescence microplate reader | |
| Excitation | 550 nm |
| Emission | 595 nm |
| Cutoff | 570 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 19, 2026

