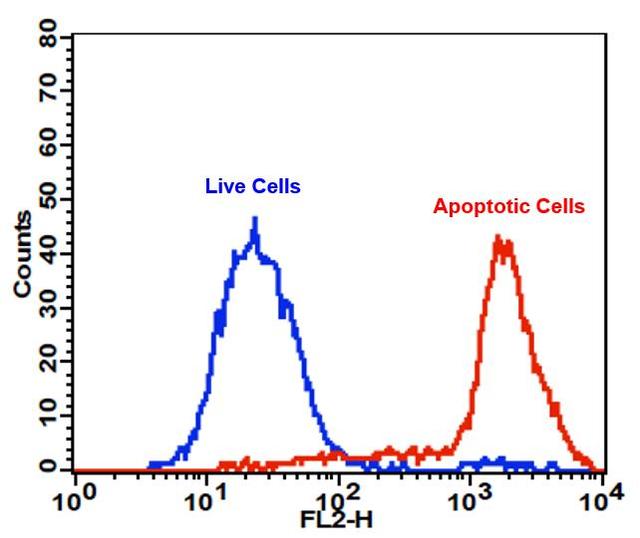
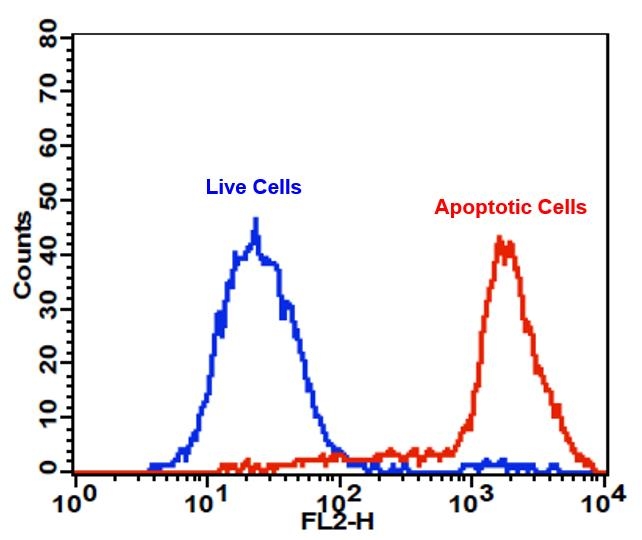
Cell Meter™ PE-Annexin V Binding Apoptosis Assay Kit *Optimized for Flow Cytometry*
Annexin V may be conjugated to fluorochromes including PE. This format retains its high affinity for phosphatidylserine (PS) and thus serves as a sensitive probe for flow cytometric analysis of cells that are undergoing apoptosis. Since externalization of PS occurs in the earlier stages of apoptosis, PE Annexin V staining can identify apoptosis at an earlier stage than assays based on nuclear changes such as DNA fragmentation. PE Annexin V staining precedes the loss of membrane integrity which accompanies the latest stages of cell death resulting from either apoptotic or necrotic processes. Therefore, staining with PE Annexin V is typically used in conjunction with a vital dye such as propidium iodide (PI) or 7-Amino-Actinomycin (7-AAD) to allow the investigator to identify early apoptotic cells (7-AAD negative, PE Annexin V positive). Viable cells with intact membranes exclude 7-AAD, whereas the membranes of dead and damaged cells are permeable to 7-AAD. For example, cells that are considered viable are both PE Annexin V and 7-AAD negative while cells that are in early apoptosis are PE Annexin V positive and 7-AAD negative, while cells that are in late apoptosis or already dead are both PE Annexin V and 7-AAD positive. This assay does not distinguish between cells that have undergone apoptotic death versus those that have died as a result of a necrotic pathway because in either case, the dead cells will stain with both PE Annexin V and 7-AAD. However, when apoptosis is measured over time, cells can be often tracked from PE Annexin V and 7-AAD negative (viable, or no measurable apoptosis), to PE Annexin V positive and 7-AAD negative (early apoptosis, membrane integrity is present) and finally to PE Annexin V and 7-AAD positive (end stage apoptosis and death). The movement of cells through these three stages suggests apoptosis. In contrast, a single observation indicating that cells are both PE Annexin V and 7-AAD positive, in of itself, reveals less information about the process by which the cells underwent their demise.
Example protocol
AT A GLANCE
Protocol summary
- Prepare cells with test compounds (200 µL/sample)
- Add RPE-Annexin V assay solution
- Incubate at room temperature for 20 - 60 minutes
- Analyze cells with a flow cytometer using 575/26 nm filter (PE channel)
PREPARATION OF STOCK SOLUTION
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles.
1. RPE-Annexin V stock solution (100X):
Add 200 µL PBS with 0.2% BSA into the vial of RPE-Annexin V (Component A) to make 100X RPE-Annexin V stock solution. Note: Store the reconstituted 100X RPE-Annexin V stock solution at 4°C. Do Not Freeze.
For guidelines on cell sample preparation, please visit
https://www.aatbio.com/resources/guides/cell-sample-preparation.html
SAMPLE EXPERIMENTAL PROTOCOL
- Treat cells with test compounds for a desired period of time (4 - 6 hours for Jurkat cells treated with staurosporine) to induce apoptosis. Note: Annexin V flow cytometric analysis on adherent cells is not routinely tested since specific membrane damage may occur during cell detachment or harvesting. However, methods for utilizing Annexin V for flow cytometry on adherent cell types have been previously reported by Casiola-Rosen et al. and van Engelend et al.
- Centrifuge the cells to get 1 - 5 × 105 cells/tube.
- Resuspend cells in 200 µL of Assay Buffer (Component B).
- Add 2 µL of 100X RPE-Annexin V stock solution into the cells.
- Optional: Add 2 µL of 100X Nuclear Red™ DCS (Component C) for necrosis cells.
- Incubate at room temperature for 20 to 60 minutes, protected from light.
- Optional: add 200 to 300 µL of Assay Buffer (Component B) to increase volume before analyzing the cells with a flow cytometer.
- Monitor the fluorescence intensity of RPE-Annexin V using a flow cytometer with 575/26 nm filter (PE channel). Measure the cell viability using 660/20 nm filter (APC channel) when Nuclear Red™ DCS is added into the cells.
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (280 nm) |
| Cell Meter™ APC-Annexin V Binding Apoptosis Assay Kit *Optimized for Flow Cytometry* | 651 | 660 | 730000 | - | 0.195 |
| Cell Meter™ FITC-Annexin V Binding Apoptosis Assay Kit *Optimized for Flow Cytometry* | 491 | 516 | 73000 | 0.92 | 0.35 |
Citations
View all 1 citations: Citation Explorer
Heparanase induces necroptosis of microvascular endothelial cells to promote the metastasis of hepatocellular carcinoma
Authors: Chen, Xiaopeng and Cheng, Bin and Dai, Dafei and Wu, Yuhai and Feng, Zhiwen and Tong, Chaogang and Wang, Xiangming and Zhao, Jun
Journal: Cell death discovery (2021): 1--13
Authors: Chen, Xiaopeng and Cheng, Bin and Dai, Dafei and Wu, Yuhai and Feng, Zhiwen and Tong, Chaogang and Wang, Xiangming and Zhao, Jun
Journal: Cell death discovery (2021): 1--13
References
View all 135 references: Citation Explorer
Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis
Authors: Mirnikjoo B, Balasubramanian K, Schroit AJ.
Journal: J Biol Chem (2009): 22512
Authors: Mirnikjoo B, Balasubramanian K, Schroit AJ.
Journal: J Biol Chem (2009): 22512
Peptidic targeting of phosphatidylserine for the MRI detection of apoptosis in atherosclerotic plaques
Authors: Burtea C, Laurent S, Lancelot E, Ballet S, Murariu O, Rousseaux O, Port M, V and er Elst L, Corot C, Muller RN.
Journal: Mol Pharm (2009): 1903
Authors: Burtea C, Laurent S, Lancelot E, Ballet S, Murariu O, Rousseaux O, Port M, V and er Elst L, Corot C, Muller RN.
Journal: Mol Pharm (2009): 1903
Apoptosis of human Burkitt's lymphoma cells induced by 2-N,N-diethylaminocarbonyloxymethyl-1-diphenylmethyl-4-(3,4,5-trimethoxybe nzoyl) piperazine hydrochloride (PMS-1077)
Authors: Wang WD, Xu XM, Chen Y, Jiang P, Dong CZ, Wang Q.
Journal: Arch Pharm Res (2009): 1727
Authors: Wang WD, Xu XM, Chen Y, Jiang P, Dong CZ, Wang Q.
Journal: Arch Pharm Res (2009): 1727
Induction of apoptosis in sonoporation and ultrasonic gene transfer
Authors: Miller DL, Dou C.
Journal: Ultrasound Med Biol (2009): 144
Authors: Miller DL, Dou C.
Journal: Ultrasound Med Biol (2009): 144
Detection of apoptosis based on the interaction between annexin V and phosphatidylserine
Authors: Liu T, Zhu W, Yang X, Chen L, Yang R, Hua Z, Li G.
Journal: Anal Chem (2009): 2410
Authors: Liu T, Zhu W, Yang X, Chen L, Yang R, Hua Z, Li G.
Journal: Anal Chem (2009): 2410
Page updated on September 19, 2024