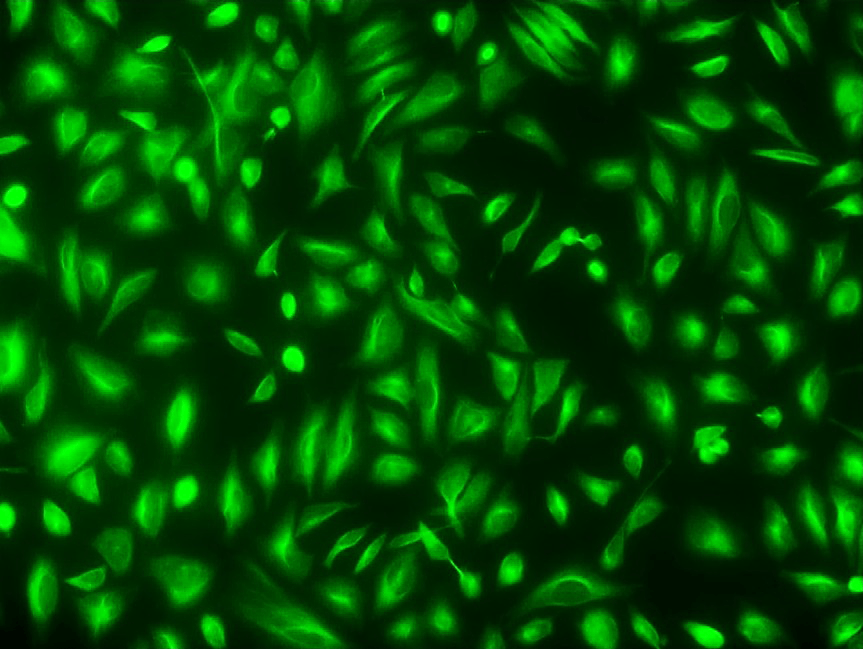
Cell Navigator® Live Cell Tubulin Staining Kit
Green Fluorescence
Cell Navigator® Live Cell Tubulin Staining Kit is ideal for high-resolution, live-cell tubulin imaging in research and drug discovery applications.
- Bright green fluorescence: Produces a bright signal for sharp and detailed microtubule visualization
- Live-cell compatibility: Allows microtubule imaging without fixation or permeabilization
- Long-lasting signal: Provides stable intracellular labeling for extended observation periods
- Applications: Suitable for studying cytoskeletal organization, tubulin polymerization, and drug-induced microtubule changes in live cells
- Comparable alternative: Offers a non-toxic live-cell tubulin staining option compared to SiR-tubulin kits from Cytoskeleton

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 23172 | 100 slides | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Instrument settings
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on December 7, 2025
