Protonex™ Red 670 acid
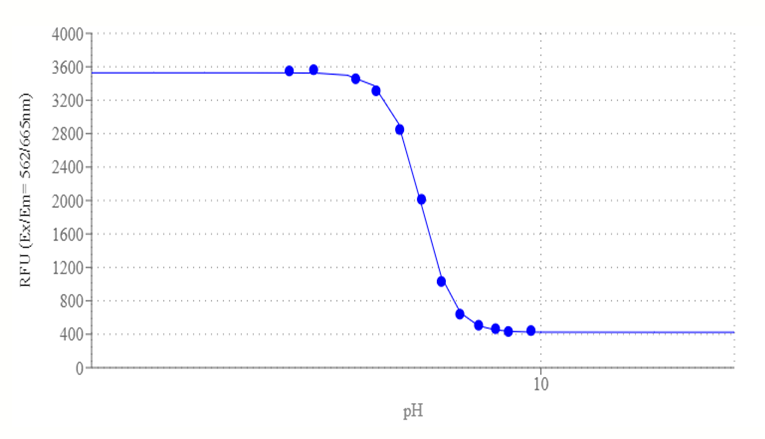
Protonex™ Red 670 works by changing its fluorescence intensity depending on the pH of the environment. Protonex™ Red 670 is minimally fluorescent at a basic pH and maximally fluorescent at an acidic pH. When Protonex™ Red 670 is bound to a receptor or an antibody on the cell surface, it is essentially non-fluorescent because the extracellular pH is neutral. However, when the receptor or antibody is internalized into the cell in response to a stimulus, it enters the endosomal pathway, where the pH is acidic. This causes Protonex™ Red 670 to become highly fluorescent and emit red light when excited by a red laser such as a 632 nm He-Ne or 647 nm red laser. By measuring the fluorescence intensity of Protonex™ Red 670, one can monitor the activation and trafficking of receptors or antibodies in live cells. Protonex™ Red 670 is especially useful in studying the activation and trafficking of G protein-coupled receptors (GPCRs), one of the most popular therapeutic drug targets. Protonex™ Red 670 can be used to label any receptor or epitope tag antibody and monitor its movement from the cell surface into acidic endosomes upon agonist stimulation. Protonex™ Red 670 can also be used to measure high-potency agonist and antagonist responses of different GPCRs in live cells, such as the activation of TRHR-1 and beta-adrenoceptor, two GPCRs that are involved in hormone regulation and cardiovascular function.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21181 | 1 mg | Price |
Physical properties
| Molecular weight | 629.76 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 643 |
| Emission (nm) | 660 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 26, 2026

