ReadiLink™ Protein Biotinylation Kit
Powered by ReadiView™ Biotin Visionization Technology
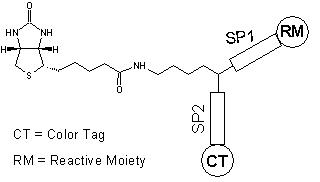
Biotin is widely used for labeling biomolecules, in particular, antibodies. This kit is primarily used for the preparation of biotin-labeled IgG for enzyme immunoassay (EIA). Our kit uses our ReadiView™ biotin succinimidyl ester (#3059) that reacts with an amino group of IgG and other biomolecules. Our unique biotin contained in the kit carries a color tag for indicating the degree of biotinylation, thus eliminating the troublesome HABA biotinylation determination. This kit contains all of the necessary reagents for labeling and purification. On our hands, 5 to 8 biotin molecules can be conjugated to each IgG molecule using our kit.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5521 | 3 Reactions | Price |
Spectral properties
| Correction factor (280 nm) | 0.05 |
| Extinction coefficient (cm -1 M -1) | 15000 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 3, 2026

