ReadiUse™ Rapid Luminometric ATP Assay Kit
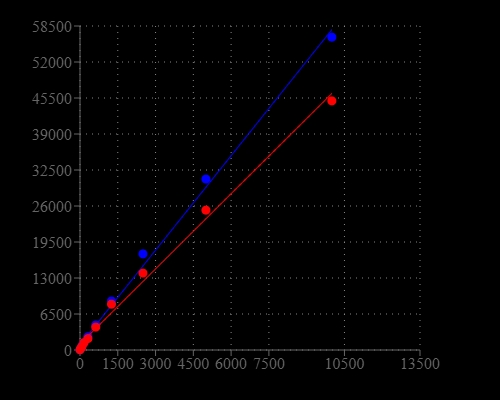
Adenosine triphosphate (ATP) plays a fundamental role in cellular energetics, metabolic regulation and cellular signaling. The quantitation of ATP can be used for a variety of biological applications. Because ATP is the energy source for almost all living organisms that rapidly degrades in the absence of viable organisms, its existence can be used to identify the presence of viable organisms. The measurement of ATP has been used for cell cytotoxicity, detection of bacteria on surfaces, quantification of bacteria in water, somatic cells in culture and food quality. The use of firefly bioluminescence to measure ATP was first proposed by McElroy when he discovered that ATP was essential for light production. Firefly luciferase is a monomeric 61 kD enzyme that catalyzes a two-step oxidation of luciferin, which yields light at 560 nm. The first step involves the activation of the protein by ATP to produce a reactive mixed anhydride intermediate. In the second step, the active intermediate reacts with oxygen to create a transient dioxetane, which quickly breaks down to the oxidized product oxyluciferin and carbon dioxide along with a burst of light. When ATP is the limiting component, the intensity of light is proportional to the concentration of ATP. Thus the measurement of the light intensity can be used for quantifying ATP using a luminometer. AAT Bioquest's ReadiUse™ Rapid Luminometric ATP Assay Kit (DTT free) comes with all the essential components in a ready-to-use format. It provides a fast, simple and homogeneous luminescence assay for monitoring cell proliferation and cytotoxicity in mammalian cells. This assay is based on the detection of ATP using firefly luciferase to catalyze the release of light by ATP and luciferin. It can be performed in a convenient 96-well or 384-well microtiter-plate format on a chemiluminescent microplate reader. The assay is extremely sensitive and can detect 50 cells/well. It has stable luminescent signal with half-life more than 2 hours. This ReadiUse™ Rapid Luminometric ATP Assay Kit does not use DTT, which eliminating the unpleasant odor.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21601 | 100 Tests | Price | |
| 21602 | 1000 Tests | Price | |
| 21603 | 10000 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Luminescence microplate reader | |
| Recommended plate | Solid white |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026
