Tide Fluor™ 3 maleimide
TF3 maleimide; Superior replacement for Cy3
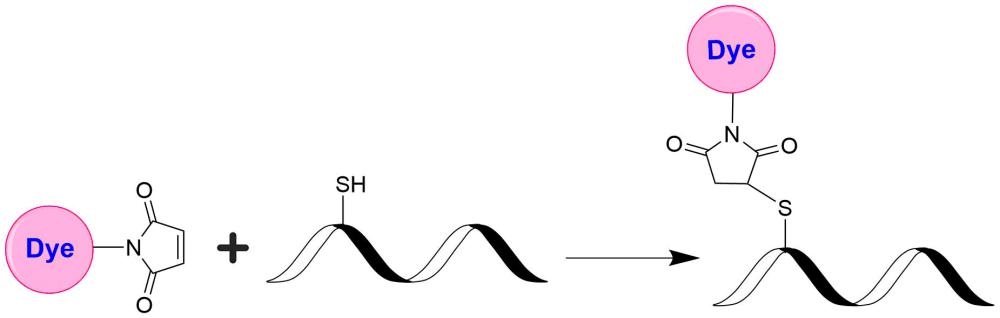
Tide Fluor™ 3 (TF3) family has the spectral properties essentially identical to those of Cy3. Compared to Cy3 probes TF3 family has much stronger fluorescence and higher photostability. Additionally their fluorescence is pH-independent from pH 3 to 11. These characteristics make this new dye family a superior alternative to Cy3. TF3-labeled peptides and nucleotides exhibit much stronger fluorescence and higher photostability than the ones labeled with Cy3. In pairing with our Tide Quencher™ 3 (TQ3), a variety of FRET peptides and nucleotides can be developed for detecting proteases and molecular beacons with enhanced sensitivity and stability. This TF3 product is used for post-labeling of thiol-modified oligonucleotides and peptides that contain cysteines.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2270 | 1 mg | Price |
Physical properties
| Molecular weight | 651.71 |
| Solvent | DMSO |
Spectral properties
| Correction factor (280 nm) | 0.179 |
| Extinction coefficient (cm -1 M -1) | 75000 1 |
| Excitation (nm) | 553 |
| Emission (nm) | 578 |
| Quantum yield | 0.1 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

