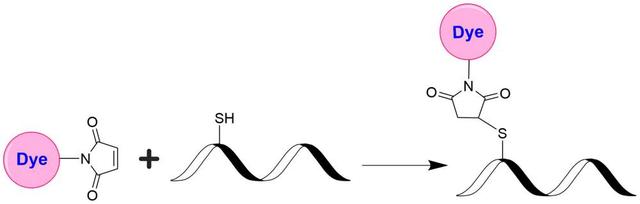
Tide Fluor™ 3 maleimide [TF3 maleimide] *Superior replacement for Cy3*
Tide Fluor™ 3 (TF3) family has the spectral properties essentially identical to those of Cy3. Compared to Cy3 probes TF3 family has much stronger fluorescence and higher photostability. Additionally their fluorescence is pH-independent from pH 3 to 11. These characteristics make this new dye family a superior alternative to Cy3. TF3-labeled peptides and nucleotides exhibit much stronger fluorescence and higher photostability than the ones labeled with Cy3. In pairing with our Tide Quencher™ 3 (TQ3), a variety of FRET peptides and nucleotides can be developed for detecting proteases and molecular beacons with enhanced sensitivity and stability. This TF3 product is used for post-labeling of thiol-modified oligonucleotides and peptides that contain cysteines.
Calculators
Common stock solution preparation
Table 1. Volume of DMSO needed to reconstitute specific mass of Tide Fluor™ 3 maleimide [TF3 maleimide] *Superior replacement for Cy3* to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 153.442 µL | 767.212 µL | 1.534 mL | 7.672 mL | 15.344 mL |
| 5 mM | 30.688 µL | 153.442 µL | 306.885 µL | 1.534 mL | 3.069 mL |
| 10 mM | 15.344 µL | 76.721 µL | 153.442 µL | 767.212 µL | 1.534 mL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (280 nm) |
| Tide Fluor™ 1 maleimide [TF1 maleimide] *Superior replacement for EDANS* | 341 | 447 | 20000 | 0.911 | 0.187 |
| Tide Fluor™ 2 maleimide [TF2 maleimide] *Superior replacement for fluorescein* | 503 | 525 | 75000 | 0.91 | 0.09 |
| Tide Fluor™ 2WS maleimide [TF2WS Maleimide] *Superior replacement for FITC* | 491 | 516 | 75000 | 0.91 | 0.11 |
| Tide Fluor™ 3 alkyne [TF3 alkyne] | 553 | 578 | 750001 | 0.11 | 0.179 |
| Tide Fluor™ 3 azide [TF3 azide] | 553 | 578 | 750001 | 0.11 | 0.179 |
| Tide Fluor™ 3 phosphoramidite [TF3 CEP] *Superior replacement to Cy3 phosphoramidite* | 560 | 580 | 750001 | - | - |
| Tide Fluor™ 3WS maleimide [TF3WS maleimide] *Superior replacement for Cy3* | 550 | 563 | 150000 | 0.151 | 0.079 |
| Tide Fluor™ 4 maleimide [TF4 maleimide] *Superior replacement for ROX and Texas Red* | 577 | 602 | 90000 | 0.771 | 0.436 |
| Tide Fluor™ 5WS maleimide [TF5WS maleimide] *Superior replacement for Cy5* | 649 | 663 | 250000 | 0.271 | 0.027 |
Show More (4) | |||||
Citations
View all 11 citations: Citation Explorer
To what extent do fluorophores bias the biological activity of peptides? A practical approach using membrane-active peptides as models
Authors: Cavaco, Marco and P{\'e}rez-Peinado, Clara and Valle, Javier and Silva, R{\'u}ben DM and Correia, Jo{\~a}o DG and Andreu, David and Castanho, Miguel ARB and Neves, Vera
Journal: Frontiers in bioengineering and biotechnology (2020): 552035
Authors: Cavaco, Marco and P{\'e}rez-Peinado, Clara and Valle, Javier and Silva, R{\'u}ben DM and Correia, Jo{\~a}o DG and Andreu, David and Castanho, Miguel ARB and Neves, Vera
Journal: Frontiers in bioengineering and biotechnology (2020): 552035
RAB33B recruits the ATG16L1 complex to the phagophore via a noncanonical RAB binding protein
Authors: Pantoom, Supansa and Konstantinidis, Georgios and Voss, Stephanie and Han, Hongmei and Hofnagel, Oliver and Li, Zhiyu and Wu, Yao-Wen
Journal: Autophagy (2020): 1--15
Authors: Pantoom, Supansa and Konstantinidis, Georgios and Voss, Stephanie and Han, Hongmei and Hofnagel, Oliver and Li, Zhiyu and Wu, Yao-Wen
Journal: Autophagy (2020): 1--15
A mechanistic model to predict effects of cathepsin B and cystatin C on β-amyloid aggregation and degradation
Authors: Perlenfein, Tyler J and Murphy, Regina M
Journal: Journal of Biological Chemistry (2017): jbc--M117
Authors: Perlenfein, Tyler J and Murphy, Regina M
Journal: Journal of Biological Chemistry (2017): jbc--M117
Using a Specific RNA--Protein Interaction To Quench the Fluorescent RNA Spinach
Authors: Roszyk, Laura and Kollenda, Sebastian and Hennig, Sven
Journal: ACS chemical biology (2017): 2958--2964
Authors: Roszyk, Laura and Kollenda, Sebastian and Hennig, Sven
Journal: ACS chemical biology (2017): 2958--2964
Spatiotemporal imaging of small GTPases activity in live cells
Authors: Voss, Stephanie and Kr{\"u}ger, Dennis M and Koch, Oliver and Wu, Yao-Wen
Journal: Proceedings of the National Academy of Sciences (2016): 14348--14353
Authors: Voss, Stephanie and Kr{\"u}ger, Dennis M and Koch, Oliver and Wu, Yao-Wen
Journal: Proceedings of the National Academy of Sciences (2016): 14348--14353
References
View all 25 references: Citation Explorer
Photodynamic molecular beacon triggered by fibroblast activation protein on cancer-associated fibroblasts for diagnosis and treatment of epithelial cancers
Authors: Lo PC, Chen J, Stefflova K, Warren MS, Navab R, B and archi B, Mullins S, Tsao M, Cheng JD, Zheng G.
Journal: J Med Chem (2009): 358
Authors: Lo PC, Chen J, Stefflova K, Warren MS, Navab R, B and archi B, Mullins S, Tsao M, Cheng JD, Zheng G.
Journal: J Med Chem (2009): 358
Time-resolved FRET method for typing polymorphic alleles of the human leukocyte antigen system by using a single DNA probe
Authors: Andreoni A, Bondani M, Nardo L.
Journal: Photochem Photobiol Sci (2009): 1202
Authors: Andreoni A, Bondani M, Nardo L.
Journal: Photochem Photobiol Sci (2009): 1202
Tumor-specific detection of an optically targeted antibody combined with a quencher-conjugated neutravidin "quencher-chaser": a dual "quench and chase" strategy to improve target to nontarget ratios for molecular imaging of cancer
Authors: Ogawa M, Kosaka N, Choyke PL, Kobayashi H.
Journal: Bioconjug Chem (2009): 147
Authors: Ogawa M, Kosaka N, Choyke PL, Kobayashi H.
Journal: Bioconjug Chem (2009): 147
The detection of platelet derived growth factor using decoupling of quencher-oligonucleotide from aptamer/quantum dot bioconjugates
Authors: Kim GI, Kim KW, Oh MK, Sung YM.
Journal: Nanotechnology (2009): 175503
Authors: Kim GI, Kim KW, Oh MK, Sung YM.
Journal: Nanotechnology (2009): 175503
Development of a cell-based hepatitis C virus infection fluorescent resonance energy transfer assay for high-throughput antiviral compound screening
Authors: Yu X, Sainz B, Jr., Uprichard SL.
Journal: Antimicrob Agents Chemother (2009): 4311
Authors: Yu X, Sainz B, Jr., Uprichard SL.
Journal: Antimicrob Agents Chemother (2009): 4311
Page updated on August 15, 2025