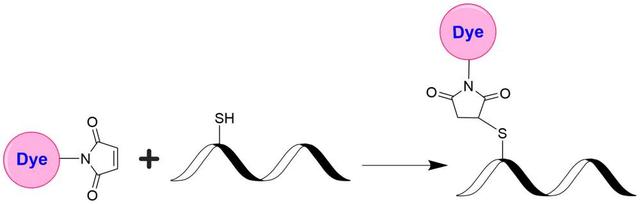
Tide Fluor™ 6WS maleimide [TF6WS maleimide] *Superior replacement for Cy5.5*
Tide Fluor™ 6WS (TF6WS) family has the spectral properties similar to those of Cy5.5, IRDye 700 and Alexa Fluor 680. Their fluorescence is pH-independent from pH 3 to 11. These characteristics make this new dye family more robust to pH-sensitive assays. In some cases TF6-labeled peptides and nucleotides exhibit stronger fluorescence and higher photostability than the ones labeled with Cy5.5, IRDye 700 and Alexa Fluor 680. In pairing with our Tide Quencher™ 6WS (TQ6WS), a variety of FRET peptides and nucleotides can be developed for detecting proteases and molecular beacons with enhanced sensitivity and stability. This TF6WS product is is used for post-labeling of thiol-modified oligonucleotides and peptides that contain cysteines.
Calculators
Common stock solution preparation
Table 1. Volume of DMSO needed to reconstitute specific mass of Tide Fluor™ 6WS maleimide [TF6WS maleimide] *Superior replacement for Cy5.5* to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 86.715 µL | 433.576 µL | 867.152 µL | 4.336 mL | 8.672 mL |
| 5 mM | 17.343 µL | 86.715 µL | 173.43 µL | 867.152 µL | 1.734 mL |
| 10 mM | 8.672 µL | 43.358 µL | 86.715 µL | 433.576 µL | 867.152 µL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (280 nm) |
| Tide Fluor™ 1 maleimide [TF1 maleimide] *Superior replacement for EDANS* | 341 | 447 | 20000 | 0.911 | 0.187 |
| Tide Fluor™ 2 maleimide [TF2 maleimide] *Superior replacement for fluorescein* | 503 | 525 | 75000 | 0.91 | 0.09 |
| Tide Fluor™ 2WS maleimide [TF2WS Maleimide] *Superior replacement for FITC* | 491 | 516 | 75000 | 0.91 | 0.11 |
| Tide Fluor™ 3 maleimide [TF3 maleimide] *Superior replacement for Cy3* | 553 | 578 | 750001 | 0.11 | 0.179 |
| Tide Fluor™ 3WS maleimide [TF3WS maleimide] *Superior replacement for Cy3* | 550 | 563 | 150000 | 0.151 | 0.079 |
| Tide Fluor™ 4 maleimide [TF4 maleimide] *Superior replacement for ROX and Texas Red* | 577 | 602 | 90000 | 0.771 | 0.436 |
| Tide Fluor™ 5WS maleimide [TF5WS maleimide] *Superior replacement for Cy5* | 649 | 663 | 250000 | 0.271 | 0.027 |
| Tide Fluor™ 6WS alkyne [TF6WS alkyne] | 682 | 701 | 220000 | 0.271 | 0.101 |
| Tide Fluor™ 6WS azide [TF6WS azide] | 682 | 701 | 220000 | 0.271 | 0.101 |
Show More (3) | |||||
Citations
View all 7 citations: Citation Explorer
A mechanistic model to predict effects of cathepsin B and cystatin C on β-amyloid aggregation and degradation
Authors: Perlenfein, Tyler J and Murphy, Regina M
Journal: Journal of Biological Chemistry (2017): jbc--M117
Authors: Perlenfein, Tyler J and Murphy, Regina M
Journal: Journal of Biological Chemistry (2017): jbc--M117
Real-Time Detection of a Self-Replicating RNA Enzyme
Authors: Olea, Charles and Joyce, Gerald F
Journal: Molecules (2016): 1310
Authors: Olea, Charles and Joyce, Gerald F
Journal: Molecules (2016): 1310
Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia
Authors: Rasanen, Juha and Quinn, Matthew J and Laurie, Amber and Bean, Eric and Roberts, Charles T and Nagalla, Srinivasa R and Gravett, Michael G
Journal: American journal of obstetrics and gynecology (2015): 82--e1
Authors: Rasanen, Juha and Quinn, Matthew J and Laurie, Amber and Bean, Eric and Roberts, Charles T and Nagalla, Srinivasa R and Gravett, Michael G
Journal: American journal of obstetrics and gynecology (2015): 82--e1
Development of Multi-Parametric/Multimodal Spectroscopy Apparatus for Characterization of Functional Interfaces
Authors: Zhou, Lang and Arugula, Mary and Easley, Christopher J and Shannon, Curtis and Simonian, Aleks and r, undefined
Journal: ECS Transactions (2015): 9--16
Authors: Zhou, Lang and Arugula, Mary and Easley, Christopher J and Shannon, Curtis and Simonian, Aleks and r, undefined
Journal: ECS Transactions (2015): 9--16
Array of biodegradable microrafts for isolation and implantation of living, adherent cells
Authors: Wang, Yuli and Phillips, Colleen N and Herrera, Gabriela S and Sims, Christopher E and Yeh, Jen Jen and Allbritton, Nancy L
Journal: RSC advances (2013): 9264--9272
Authors: Wang, Yuli and Phillips, Colleen N and Herrera, Gabriela S and Sims, Christopher E and Yeh, Jen Jen and Allbritton, Nancy L
Journal: RSC advances (2013): 9264--9272
References
View all 25 references: Citation Explorer
Evaluation of tetramethylrhodamine and black hole quencher 1 labeled probes and five commercial amplification mixes in TaqMan real-time RT-PCR assays for respiratory pathogens
Authors: Yang GP, Erdman DD, Tondella ML, Fields BS.
Journal: J Virol Methods (2009): 288
Authors: Yang GP, Erdman DD, Tondella ML, Fields BS.
Journal: J Virol Methods (2009): 288
Time-resolved FRET method for typing polymorphic alleles of the human leukocyte antigen system by using a single DNA probe
Authors: Andreoni A, Bondani M, Nardo L.
Journal: Photochem Photobiol Sci (2009): 1202
Authors: Andreoni A, Bondani M, Nardo L.
Journal: Photochem Photobiol Sci (2009): 1202
Tumor-specific detection of an optically targeted antibody combined with a quencher-conjugated neutravidin "quencher-chaser": a dual "quench and chase" strategy to improve target to nontarget ratios for molecular imaging of cancer
Authors: Ogawa M, Kosaka N, Choyke PL, Kobayashi H.
Journal: Bioconjug Chem (2009): 147
Authors: Ogawa M, Kosaka N, Choyke PL, Kobayashi H.
Journal: Bioconjug Chem (2009): 147
The detection of platelet derived growth factor using decoupling of quencher-oligonucleotide from aptamer/quantum dot bioconjugates
Authors: Kim GI, Kim KW, Oh MK, Sung YM.
Journal: Nanotechnology (2009): 175503
Authors: Kim GI, Kim KW, Oh MK, Sung YM.
Journal: Nanotechnology (2009): 175503
Development of a cell-based hepatitis C virus infection fluorescent resonance energy transfer assay for high-throughput antiviral compound screening
Authors: Yu X, Sainz B, Jr., Uprichard SL.
Journal: Antimicrob Agents Chemother (2009): 4311
Authors: Yu X, Sainz B, Jr., Uprichard SL.
Journal: Antimicrob Agents Chemother (2009): 4311
Page updated on August 27, 2025