HIS Lite™ Cy5 Tris NTA-Ni Complex
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | 2168.75 |
| Solvent | Water |
| Correction Factor (260 nm) | 0.02 |
| Correction Factor (280 nm) | 0.03 |
| Correction Factor (482 nm) | 0.009 |
| Correction Factor (565 nm) | 0.09 |
| Extinction coefficient (cm -1 M -1) | 2500001 |
| Excitation (nm) | 651 |
| Emission (nm) | 670 |
| Quantum yield | 0.271, 0.42 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| HIS Lite™ iFluor™ 647 Tris NTA-Ni Complex |
| Overview |
Molecular weight 2168.75 | Correction Factor (260 nm) 0.02 | Correction Factor (280 nm) 0.03 | Correction Factor (482 nm) 0.009 | Correction Factor (565 nm) 0.09 | Extinction coefficient (cm -1 M -1) 2500001 | Excitation (nm) 651 | Emission (nm) 670 | Quantum yield 0.271, 0.42 |
Platform
Gel Imager
| Excitation | Red laser |
| Emission | 700/50 nm |
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 5 to 10 mM stock solution by adding an appropriate amount of DMSO.
Note: Store any unused stock solution at -20 °C. Avoid repeated freeze-thaw cycles and minimize light exposure.
PREPARATION OF WORKING SOLUTION
Prepare a 1 to 10 µM HIS Lite™ Cy5 Tris NTA-Ni Complex working solution in PBS.
Note: Ensure that there is sufficient working solution to fully submerge the gel. After use, discard the working solution. Do not reuse.
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol should be used only as a guideline and may require optimization to better suit your specific experimental needs.
Run gels based on your standard protocol.
Place the gel in a suitable container. Fix the gel in the fixing solution for 60 minutes. Note: 40% ethanol + 10% acetic acid can be used as a fixing solution.
Wash the gel twice with the ultra-pure water.
Incubate the gel in the HIS Lite™ Cy5 Tris NTA-Ni Complex working solution for 60 minutes.
Note: Be sure to fully submerge the gel in the working solution.
Remove the working solution and wash the gel twice with PBS.
Proceed to imaging the gel immediately.
Mix the His-tagged protein solution and the HIS Lite™ Cy5 Tris NTA-Ni Complex working solution at the appropriate concentrations.
Note: Optimization of the HIS Lite™ Cy5 Tris NTA-Ni Complex to the His-tagged protein mix must be performed for better labeling.
Note: 1 to 10 µM of HIS Lite™ Cy5 Tris NTA-Ni Complex can be used as a starting concentration.
Note: The reaction can be performed in a buffer containing 50 mM HEPES/KOH, pH 7.4, 100 mM KCl, 1 mM MgCl2, 2 mM β-mercaptoethanol, 5% glycerol, or a buffer of your choice.
Mix can be incubated for 30 minutes at room temperature or 4 ℃.
Note: Optimization of the incubation time and conditions must be performed for better labeling
Mix can then be subjected to column purification or any other downstream process.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 46.11 µL | 230.548 µL | 461.095 µL | 2.305 mL | 4.611 mL |
| 5 mM | 9.222 µL | 46.11 µL | 92.219 µL | 461.095 µL | 922.19 µL |
| 10 mM | 4.611 µL | 23.055 µL | 46.11 µL | 230.548 µL | 461.095 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
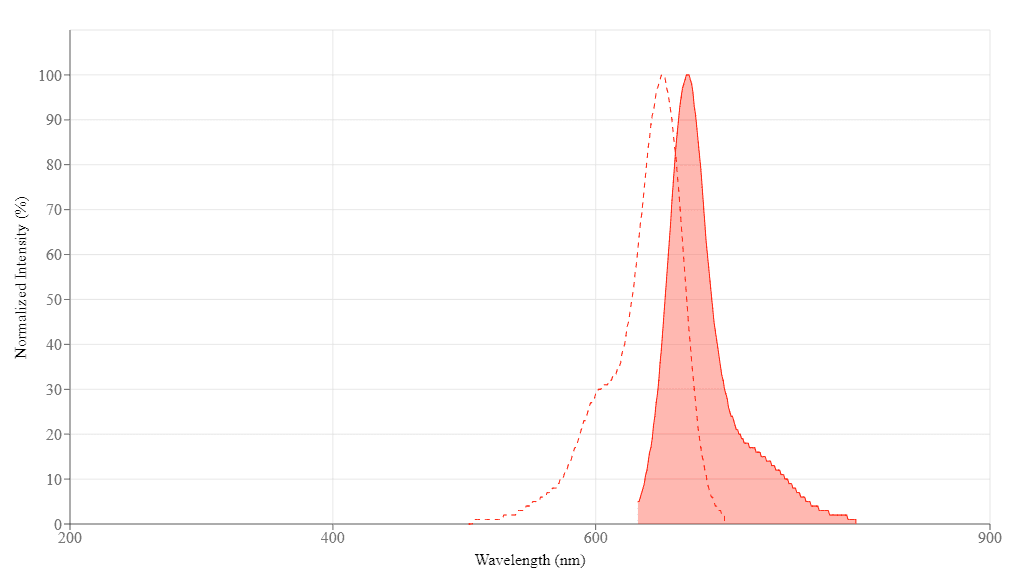
Spectral properties
| Correction Factor (260 nm) | 0.02 |
| Correction Factor (280 nm) | 0.03 |
| Correction Factor (482 nm) | 0.009 |
| Correction Factor (565 nm) | 0.09 |
| Extinction coefficient (cm -1 M -1) | 2500001 |
| Excitation (nm) | 651 |
| Emission (nm) | 670 |
| Quantum yield | 0.271, 0.42 |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| HIS Lite™ Cy5 Bis NTA-Ni Complex | 651 | 670 | 2500001 | 0.271, 0.42 | 0.02 | 0.03 |
| HIS Lite™ OG488-Tris NTA-Ni Complex | 498 | 526 | 76000 | - | 0.31 | 0.12 |
| HIS Lite™ Cy3 Tris NTA-Ni Complex | 555 | 569 | 1500001 | 0.151 | 0.07 | 0.073 |
Images

References
Authors: Song, Jiaping and Zhou, Chuanwen and Liu, Rui and Wu, Xudong and Wu, Di and Hu, Xiaojian and Ding, Yu
Journal: Molecular biology reports (2010): 1823-9
Authors: Li, Bei and Ji, Lingling and Wu, Yunfeng and Hao, Xing'an
Journal: Wei sheng wu xue bao = Acta microbiologica Sinica (2008): 739-44
Authors: Jonák, J
Journal: Journal of chromatography. B, Analytical technologies in the biomedical and life sciences (2007): 141-53
Authors: Wang, Li and Sun, Ming and Yu, Zi Niu
Journal: Shi yan sheng wu xue bao (2003): 476-81
Authors: Jouanneau, E and Alberti, L and Nejjari, M and Treilleux, I and Vilgrain, I and Duc, A and Combaret, V and Favrot, M and Leboulch, P and Bachelot, T
Journal: Journal of neuro-oncology (2001): 11-8
Authors: Müller, S and Adomeit, A and Kaufmann, R and Appelhans, H and Passow, H and Reissmann, S and Liebmann, C
Journal: Biological chemistry (2000): 343-7
Authors: Lockhart, E and Griffin, J F and Chinn, N and Lavallie, E and Buchan, G
Journal: Cytokine (1996): 603-12
Authors: Fukuoka, Y and Tachibana, T and Yasui, A
Journal: Immunology letters (1993): 153-8
Authors: Chen, Hsiu-Mei and Wang, Wei-Cheng and Chen, Sheng-Horng
Journal: Biotechnology progress : 1237-44