HIS Lite™ iFluor® 568 Tris NTA-Ni Complex
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | 1936.37 |
| Solvent | Water |
| Correction Factor (260 nm) | 0.34 |
| Correction Factor (280 nm) | 0.15 |
| Extinction coefficient (cm -1 M -1) | 1000001 |
| Excitation (nm) | 568 |
| Emission (nm) | 587 |
| Quantum yield | 0.571 |
| Intended use | Research Use Only (RUO) |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| Overview |
Molecular weight 1936.37 | Correction Factor (260 nm) 0.34 | Correction Factor (280 nm) 0.15 | Extinction coefficient (cm -1 M -1) 1000001 | Excitation (nm) 568 | Emission (nm) 587 | Quantum yield 0.571 |
Platform
Gel Imager
| Excitation | Green laser |
| Emission | 602/50 nm |
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 5 to 10 mM stock solution by adding an appropriate amount of ddH2O.
Note: Store any unused stock solution at -20 °C. Avoid repeated freeze-thaw cycles and minimize light exposure.
PREPARATION OF WORKING SOLUTION
Prepare a 1 to 10 µM HIS Lite™ iFluor® 568 Tris NTA-Ni Complex working solution in PBS.
Note: Ensure that there is sufficient working solution to fully submerge the gel. After use, discard the working solution. Do not reuse.
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol should be used only as a guideline and may require optimization to better suit your specific experimental needs.
Run gels based on your standard protocol.
Place the gel in a suitable container. Fix the gel in the fixing solution for 60 minutes. Note: 40% ethanol + 10% acetic acid can be used as a fixing solution.
Wash the gel twice with the ultra-pure water.
Incubate the gel in the HIS Lite™ iFluor® 568 Tris NTA-Ni Complex working solution for 60 minutes.
Note: Be sure to fully submerge the gel in the working solution.
Remove the working solution and wash the gel twice with PBS.
Proceed to imaging the gel immediately.
Mix the His-tagged protein solution and the HIS Lite™ iFluor® 568 Tris NTA-Ni Complex working solution at the appropriate concentrations.
Note: Optimization of the HIS Lite™ iFluor® 568 Tris NTA-Ni Complex to the His-tagged protein mix must be performed for better labeling.
Note: 1 to 10 µM of HIS Lite™ iFluor® 568 Tris NTA-Ni Complex can be used as a starting concentration.
Note: The reaction can be performed in a buffer containing 50 mM HEPES/KOH, pH 7.4, 100 mM KCl, 1 mM MgCl2, 2 mM β-mercaptoethanol, 5% glycerol, or a buffer of your choice.
Mix can be incubated for 30 minutes at room temperature or 4 ℃.
Note: Optimization of the incubation time and conditions must be performed for better labeling
Mix can then be subjected to column purification or any other downstream process.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 51.643 µL | 258.215 µL | 516.43 µL | 2.582 mL | 5.164 mL |
| 5 mM | 10.329 µL | 51.643 µL | 103.286 µL | 516.43 µL | 1.033 mL |
| 10 mM | 5.164 µL | 25.822 µL | 51.643 µL | 258.215 µL | 516.43 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
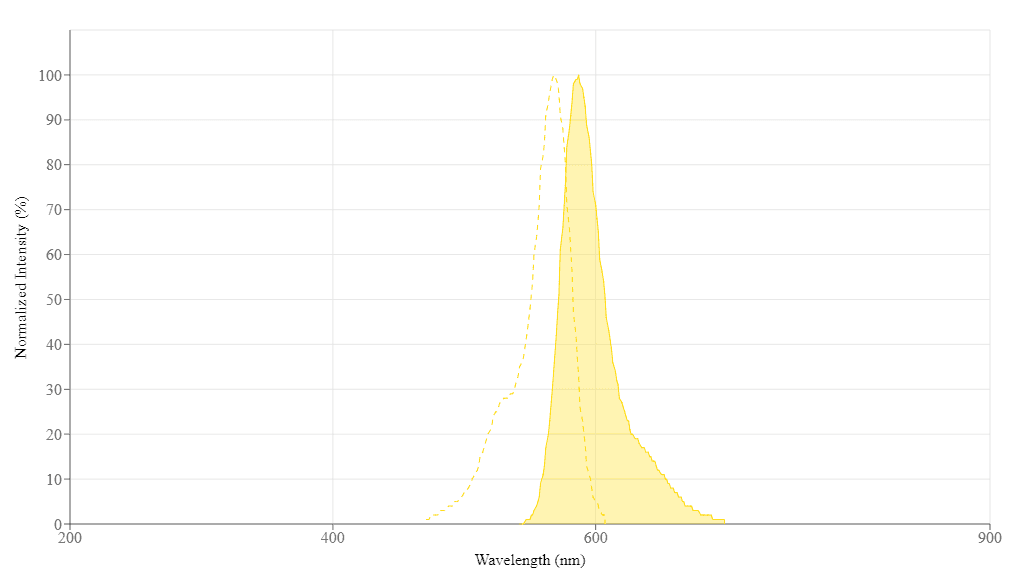
Spectral properties
| Correction Factor (260 nm) | 0.34 |
| Correction Factor (280 nm) | 0.15 |
| Extinction coefficient (cm -1 M -1) | 1000001 |
| Excitation (nm) | 568 |
| Emission (nm) | 587 |
| Quantum yield | 0.571 |
Citations
Authors: Zhou, Y., Yan, D., Yuan, S., Chen, Y., Fletcher, E. E., Shi, H., Han, B.
Journal: Protein Expr Purif (2018): 5-11
Authors: Palani, B.
Journal: Monoclon Antib Immunodiagn Immunother (2018): 87-90
Authors: Prabhu, A. A., Purkayastha, A., M and al, B., Kumar, J. P., M and al, B. B., Veeranki, V. D.
Journal: Int J Biol Macromol (2018): 2512-2524
Authors: Minamiki, T., Sasaki, Y., Tokito, S., Minami, T.
Journal: ChemistryOpen (2017): 472-475
Authors: Wasserberg, D., Cabanas-Danes, J., Prangsma, J., O'Mahony, S., Cazade, P. A., Tromp, E., Blum, C., Thompson, D., Huskens, J., Subramaniam, V., Jonkheijm, P.
Journal: ACS Nano (2017): 9068-9083
Authors: Benjamin, C. J., Wright, K. J., Hyun, S. H., Krynski, K., Yu, G., Bajaj, R., Guo, F., Stauffacher, C. V., Jiang, W., Thompson, D. H.
Journal: Langmuir (2016): 551-9
Authors: Gulka, C. P., Swartz, J. D., Wright, D. W.
Journal: Talanta (2015): 94-101
Authors: Gulka, C. P., Swartz, J. D., Trantum, J. R., Davis, K. M., Peak, C. M., Denton, A. J., Haselton, F. R., Wright, D. W.
Journal: ACS Appl Mater Interfaces (2014): 6257-63
Authors: Anthony, K. C., You, C., Piehler, J., Pomeranz Krummel, D. A.
Journal: Structure (2014): 628-35
Authors: Fischer, M., Leech, A. P., Hubbard, R. E.
Journal: Anal Chem (2011): 1800-7
