HIS Lite™ Cy3 Tris NTA-Ni Complex
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | 2142.71 |
| Solvent | Water |
| Correction Factor (260 nm) | 0.07 |
| Correction Factor (280 nm) | 0.073 |
| Extinction coefficient (cm -1 M -1) | 1500001 |
| Excitation (nm) | 555 |
| Emission (nm) | 569 |
| Quantum yield | 0.151 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| Overview |
Molecular weight 2142.71 | Correction Factor (260 nm) 0.07 | Correction Factor (280 nm) 0.073 | Extinction coefficient (cm -1 M -1) 1500001 | Excitation (nm) 555 | Emission (nm) 569 | Quantum yield 0.151 |
Platform
Gel Imager
| Excitation | Green laser |
| Emission | 602/50 nm |
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 5 to 10 mM stock solution by adding an appropriate amount of DMSO.
Note: Store any unused stock solution at -20 °C. Avoid repeated freeze-thaw cycles and minimize light exposure.
PREPARATION OF WORKING SOLUTION
Prepare a 1 to 10 µM HIS Lite™ Cy3 Tris NTA-Ni Complex working solution in PBS.
Note: Ensure that there is sufficient working solution to fully submerge the gel. After use, discard the working solution. Do not reuse.
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol should be used only as a guideline and may require optimization to better suit your specific experimental needs.
Run gels based on your standard protocol.
Place the gel in a suitable container. Fix the gel in the fixing solution for 60 minutes. Note: 40% ethanol + 10% acetic acid can be used as a fixing solution.
Wash the gel twice with the ultra-pure water.
Incubate the gel in the HIS Lite™ Cy3 Tris NTA-Ni Complex working solution for 60 minutes.
Note: Be sure to fully submerge the gel in the working solution.
Remove the working solution and wash the gel twice with PBS.
Proceed to imaging the gel immediately.
Mix the His-tagged protein solution and the HIS Lite™ Cy3 Tris NTA-Ni Complex working solution at the appropriate concentrations.
Note: Optimization of the HIS Lite™ Cy3 Tris NTA-Ni Complex to the His-tagged protein mix must be performed for better labeling.
Note: 1 to 10 µM of HIS Lite™ Cy3 Tris NTA-Ni Complex can be used as a starting concentration.
Note: The reaction can be performed in a buffer containing 50 mM HEPES/KOH, pH 7.4, 100 mM KCl, 1 mM MgCl2, 2 mM β-mercaptoethanol, 5% glycerol, or a buffer of your choice.
Mix can be incubated for 30 minutes at room temperature or 4 ℃.
Note: Optimization of the incubation time and conditions must be performed for better labeling
Mix can then be subjected to column purification or any other downstream process.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 46.67 µL | 233.349 µL | 466.699 µL | 2.333 mL | 4.667 mL |
| 5 mM | 9.334 µL | 46.67 µL | 93.34 µL | 466.699 µL | 933.397 µL |
| 10 mM | 4.667 µL | 23.335 µL | 46.67 µL | 233.349 µL | 466.699 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
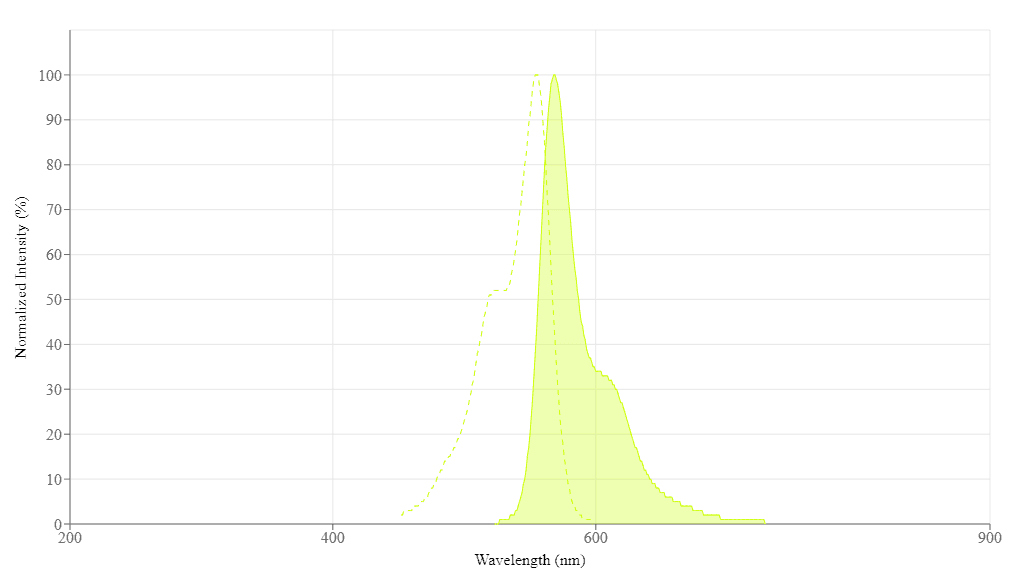
Spectral properties
| Correction Factor (260 nm) | 0.07 |
| Correction Factor (280 nm) | 0.073 |
| Extinction coefficient (cm -1 M -1) | 1500001 |
| Excitation (nm) | 555 |
| Emission (nm) | 569 |
| Quantum yield | 0.151 |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| HIS Lite™ Cy3 Bis NTA-Ni Complex | 555 | 569 | 1500001 | 0.151 | 0.07 | 0.073 |
| HIS Lite™ OG488-Tris NTA-Ni Complex | 498 | 526 | 76000 | - | 0.31 | 0.12 |
| HIS Lite™ Cy5 Tris NTA-Ni Complex | 651 | 670 | 2500001 | 0.271, 0.42 | 0.02 | 0.03 |
Images
References
Authors: Tateo, Seigo and Shinchi, Hiroyuki and Matsumoto, Hikaru and Nagata, Nonoka and Hashimoto, Masahito and Wakao, Masahiro and Suda, Yasuo
Journal: Colloids and surfaces. B, Biointerfaces (2023): 113192
Authors: Lu, Zhiwei and Li, Mengjiao and Chen, Maoting and Wang, Qirui and Wu, Chun and Sun, Mengmeng and Su, Gehong and Wang, Xianxiang and Wang, Yanying and Zhou, Xinguang and Ye, Jianshan and Liu, Tao and Rao, Hanbing
Journal: Food chemistry (2023): 135640
Authors: Zhao, Haowen and Zastrow, Melissa L
Journal: Biochemistry (2022): 494-504
Authors: Bryan, Louise and Awasthi, Saurabh and Li, Yuanjie and Nirmalraj, Peter Niraj and Balog, Sandor and Yang, Jerry and Mayer, Michael
Journal: ACS omega (2022): 47009-47014
Authors: Durner, Anna and Nicke, Annette
Journal: Methods in molecular biology (Clifton, N.J.) (2022): 193-216
Authors: Ning, Xuerao and Yasuda, Takanobu and Kitaguchi, Tetsuya and Ueda, Hiroshi
Journal: Sensors (Basel, Switzerland) (2021)
Authors: Kinoshita-Kikuta, Emiko and Kinoshita, Eiji and Koike, Tohru
Journal: Methods in molecular biology (Clifton, N.J.) (2021): 73-78
Authors: Sadoine, Mayuri and Castro-Rodríguez, Vanessa and Poloczek, Tobias and Javot, Helene and Sunal, Erdem and Wudick, Michael M and Frommer, Wolf B
Journal: Bio-protocol (2020): e3773
Authors: Brüchert, Stefan and Joest, Eike F and Gatterdam, Karl and Tampé, Robert
Journal: Communications biology (2020): 138
Authors: Kinoshita-Kikuta, Emiko and Kusamoto, Hiroshi and Ono, Syogo and Akayama, Keisuke and Eguchi, Yoko and Igarashi, Masayuki and Okajima, Toshihide and Utsumi, Ryutaro and Kinoshita, Eiji and Koike, Tohru
Journal: Electrophoresis (2019): 3005-3013
Application notes
FAQ
What is the difference between FluoroQuest Anti-fading Kit I and FluoroQuest Anti-fading Kit II?
What does an assay buffer do?
How to lyse cells?
Why is my GSH concentration greater than my Total GSH concentration? Why is my calculated GSH/TGSH ratio greater than 1?
