HIS Lite™ iFluor™ 647 Tris NTA-Ni Complex
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Molecular weight | 2094.58 |
| Solvent | Water |
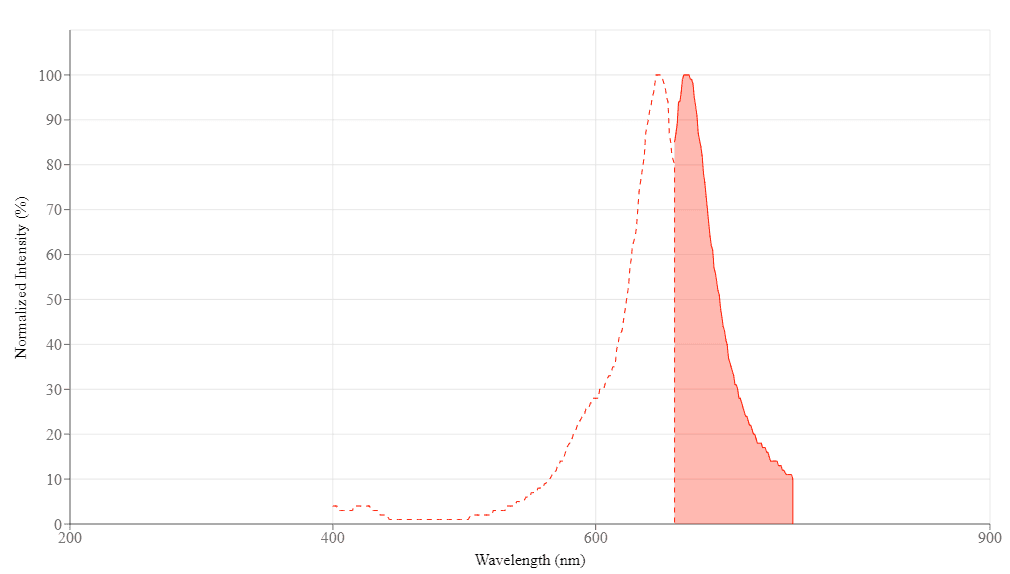
| Excitation (nm) | 648 |
| Emission (nm) | 668 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| Overview |
Molecular weight 2094.58 | Excitation (nm) 648 | Emission (nm) 668 |
Platform
Gel Imager
| Excitation | Red laser |
| Emission | 700/50 nm |
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 5 to 10 mM stock solution by adding an appropriate amount of ddH2O.
Note: Store any unused stock solution at -20 °C. Avoid repeated freeze-thaw cycles and minimize light exposure.
PREPARATION OF WORKING SOLUTION
Prepare a 1 to 10 µM HIS Lite™ iFluor® 647 Tris NTA-Ni Complex working solution in PBS.
Note: Ensure that there is sufficient working solution to fully submerge the gel. After use, discard the working solution. Do not reuse.
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol should be used only as a guideline and may require optimization to better suit your specific experimental needs.
Run gels based on your standard protocol.
Place the gel in a suitable container. Fix the gel in the fixing solution for 60 minutes. Note: 40% ethanol + 10% acetic acid can be used as a fixing solution.
Wash the gel twice with the ultra-pure water.
Incubate the gel in the HIS Lite™ iFluor® 647 Tris NTA-Ni Complex working solution for 60 minutes.
Note: Be sure to fully submerge the gel in the working solution.
Remove the working solution and wash the gel twice with PBS.
Proceed to imaging the gel immediately.
Mix the His-tagged protein solution and the HIS Lite™ iFluor® 647 Tris NTA-Ni Complex working solution at the appropriate concentrations.
Note: Optimization of the HIS Lite™ iFluor® 647 Tris NTA-Ni Complex to the His-tagged protein mix must be performed for better labeling.
Note: 1 to 10 µM of HIS Lite™ iFluor® 647 Tris NTA-Ni Complex can be used as a starting concentration.
Note: The reaction can be performed in a buffer containing 50 mM HEPES/KOH, pH 7.4, 100 mM KCl, 1 mM MgCl2, 2 mM β-mercaptoethanol, 5% glycerol, or a buffer of your choice.
Mix can be incubated for 30 minutes at room temperature or 4 ℃.
Note: Optimization of the incubation time and conditions must be performed for better labeling
Mix can then be subjected to column purification or any other downstream process.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 47.742 µL | 238.711 µL | 477.423 µL | 2.387 mL | 4.774 mL |
| 5 mM | 9.548 µL | 47.742 µL | 95.485 µL | 477.423 µL | 954.845 µL |
| 10 mM | 4.774 µL | 23.871 µL | 47.742 µL | 238.711 µL | 477.423 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| HIS Lite™ iFluor® 568 Tris NTA-Ni Complex | 568 | 587 | 1000001 | 0.571 | 0.34 | 0.15 |
Images

References
Authors: Shafifar, Mina and Mozhgani, Sayed-Hamidreza and Razavi Pashabayg, Kobra and Mosavat, Arman and Karbalaei, Mohsen and Norouzi, Mehdi and Rezaee, Seyed Abdolrahim
Journal: Life sciences (2022): 120920
Authors: Raducanu, Vlad-Stefan and Isaioglou, Ioannis and Raducanu, Daniela-Violeta and Merzaban, Jasmeen S and Hamdan, Samir M
Journal: The Journal of biological chemistry (2020): 12214-12223
Authors: Pan, Xiaoshu and Yang, Yu and Li, Long and Li, Xiaowei and Li, Qiang and Cui, Cheng and Wang, Bang and Kuai, Hailan and Jiang, Jianhui and Tan, Weihong
Journal: Chemical science (2020): 9648-9654
Authors: Yang, Hui and Xiong, Haoran and Mi, Kaihang and Zhang, Yingying and Zhang, Xiaojun and Chen, Guohong
Journal: Fish & shellfish immunology (2020): 62-68
Authors: Crauwels, Maxine and Van Vaerenbergh, Nele and Kulaya, Neeme Benedict and Vincke, Cécile and D'Huyvetter, Matthias and Devoogdt, Nick and Muyldermans, Serge and Xavier, Catarina
Journal: New biotechnology (2020): 20-28
Authors: Li, Wen-Jing and Xiang, Bei-Bei and Sun, Yan-Xiang and Hou, Xiao-Qiang and Han, Mei-Ling and Li, Xiao-Xue and Wang, Yong and Guo, Shuo
Journal: Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica (2019): 935-941
Authors: Zondagh, Jake and Williams, Alison and Achilonu, Ikechukwu and Dirr, Heini W and Sayed, Yasien
Journal: The protein journal (2018): 369-379
Authors: Huang, Jingwei and Xiong, Kang and Zhang, Houshuang and Zhao, Yanzhen and Cao, Jie and Gong, Haiyan and Zhou, Yongzhi and Zhou, Jinlin
Journal: Parasites & vectors (2018): 38
Authors: Torres-González, Lisa and Díaz-Ayala, Ramonita and Vega-Olivencia, Carmen A and López-Garriga, Juan
Journal: Sensors (Basel, Switzerland) (2018)
Authors: Rashid, Zahra and Naeimi, Hossein and Zarnani, Amir-Hassan and Mohammadi, Fereshteh and Ghahremanzadeh, Ramin
Journal: Materials science & engineering. C, Materials for biological applications (2017): 670-676