Buccutite™ Rapid PE Antibody Labeling Kit
Microscale Optimized for Labeling 100 ug Antibody Per Reaction
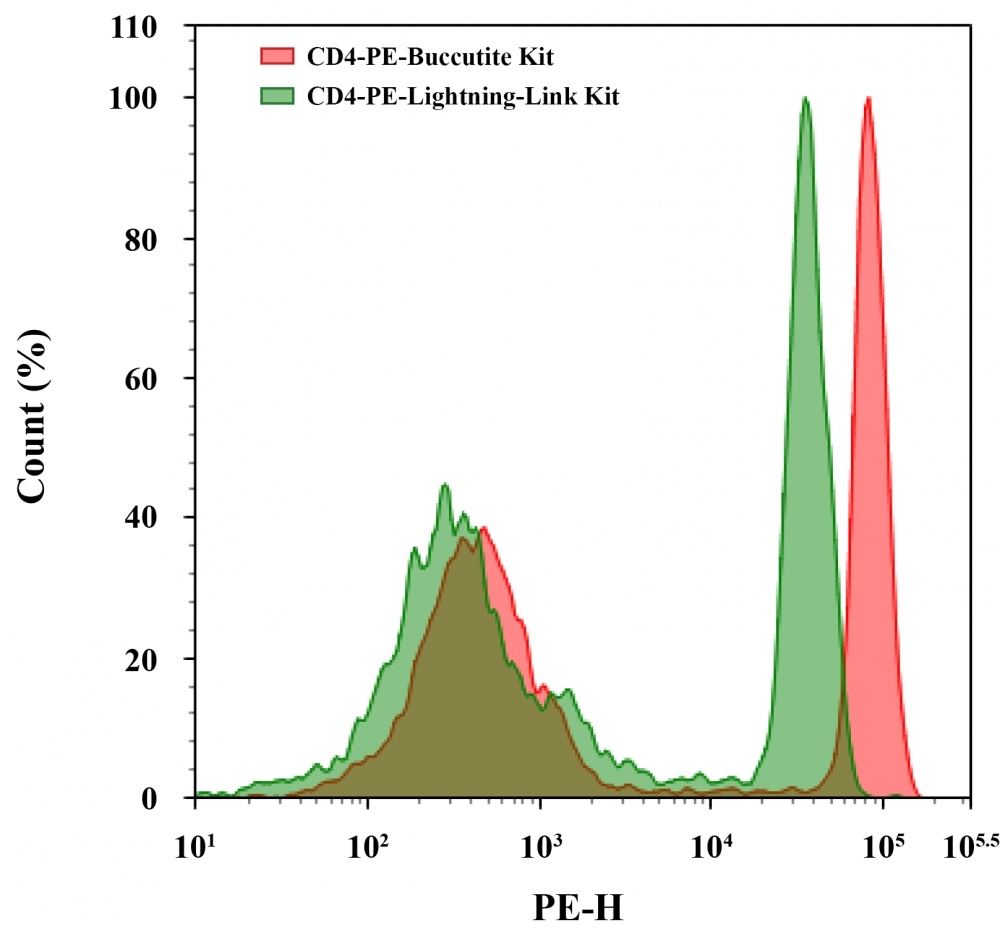
R-Phycoerythrin (PE) is an orange fluorescent protein which has an excitation wavelength of 565 nm and an emission wavelength of 575 nm. AAT Bioquest offers this Buccutite™ rapid labeling kit to facilitate the PE conjugations to antibodies and other proteins such as streptavidin and other secondary reagents. Buccutite™ PE Conjugation Kit provides a robust and convenient method to conjugate your antibodies with PE. The kit includes a preactivated PE and reaction buffer. The conjugated antibody can be used in flow cytometry, WB, ELISA and IHC applications. This kit is sufficient for 2 labeling reactions, each up to 100 ug of antibody. Considering the large size of PE (240 kDa), the amount of antibody used in a labeling reaction must always be less than the amount of PE. The best ratio for any new antibody reagent must be determined by experimentation but 50-60 ug of IgG antibody for every 100 ug of PE usually gives optimal results. Our kit provides preactivated PE to facilitate the PE conjugations to antibodies and other proteins such as streptavidin and other secondary reagents. Our preactivated PE is ready to conjugate, giving much higher yield than the conventionally tedious SMCC-based conjugation chemistry. In addition, our preactivated PE is conjugated to a protein via its amino group that is abundant in proteins while SMCC chemistry targets the thiol group that has to be regenerated by the reduction of antibodies.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1310 | 2 Labelings | Price |
Spectral properties
| Correction factor (280 nm) | 0.175 |
| Extinction coefficient (cm -1 M -1) | 1960000 |
| Excitation (nm) | 565 |
| Emission (nm) | 574 |
| Quantum yield | 0.82 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 19, 2026

