Cell Meter™ Fluorimetric Intracellular Total ROS Activity Assay Kit
Orange Fluorescence
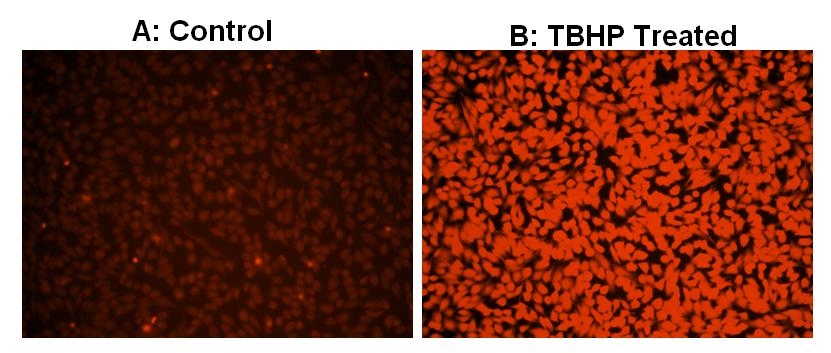
Reactive oxygen species (ROS) are natural byproducts of the normal metabolism of oxygen and play important roles in cell signaling. The accumulation of ROS results in significant damage to cell structures. The role of oxidative stress in cardiovascular disease, diabetes, osteoporosis, stroke, inflammatory diseases, a number of neurodegenerative diseases and cancer has been well established. The ROS measurement will help to determine how oxidative stress modulates varied intracellular pathways. Cell Meter™ Fluorimetric Intracellular Total ROS Activity Assay Kit uses our proprietary ROS Brite™ 570 sensor to quantify ROS in live cells. The cell-permeable and non-fluorescent ROS Brite™ 570 exhibits a strong fluorescence signal upon reaction with ROS. ROS Brite™ 570 sensor is localized in the cytoplasm. The fluorescence signal of ROS Brite™ 570 sensor can be measured by fluorescence microscopy, high-content imaging, microplate fluorometry, or flow cytometry. The Cell Meter™ Fluorimetric Intracellular Total ROS Activity Assay Kit provides a sensitive, one-step fluorimetric assay to detect intracellular ROS (especially superoxide and hydroxyl radical) in live cells within 1 hour incubation. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format using either a fluorescence microplate reader or a fluorescent microscope with TRITC filter.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22902 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm or 532 nm laser |
| Emission | 575/26 nm filter |
| Instrument specification(s) | PE channel |
| Fluorescence microscope | |
| Excitation | TRITC filter |
| Emission | TRITC filter |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 570 nm |
| Cutoff | 550 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 26, 2026
