Cell Meter™ Fluorimetric Phagocytosis Assay Kit
Green Fluorescence
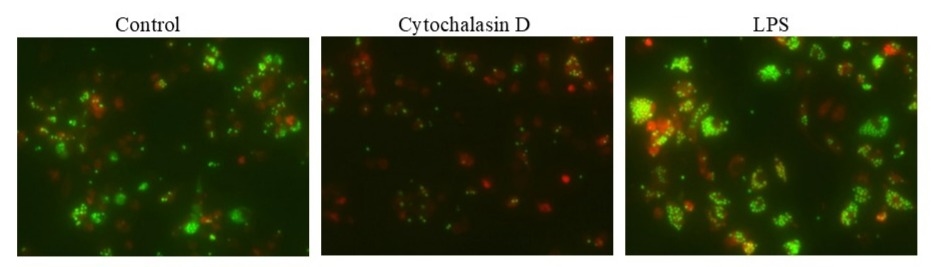
The Cell Meter™ Fluorimetric Phagocytosis Assay Kit enables robust and sensitive detection of phagocytic activity in live cells using a unique pH-sensitive fluorescent conjugate.
- pH-activated fluorescence detection: Utilizes Protonex™ Green 500-Zymosan conjugate, a pH-responsive, non-fluorescent probe that fluoresces strongly in acidic phagosomes and phagolysosomes
- Dual-color assay format: Includes a red fluorescent viability dye to simultaneously assess cell viability (red) and phagocytic function (green)
- Application versatility: Suitable for immunology research, drug screening, and cellular function analysis using microscopy, flow cytometry, or microplate formats
- Comparable alternative: Serves as a dual-fluorescence alternative to BioLegend’s Phagocytosis Detection Kit with enhanced acid-activated signal specificity

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21231 | 100 Tests | Price |
Physical properties
| Solvent |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 4000 |
| Excitation (nm) | 445 |
| Emission (nm) | 503 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C); Minimize light exposure |
Instrument settings
| Fluorescence microscope | |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | FITC/Texas Red Filters |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 28, 2026

