Buccutite™ Rapid PerCP Antibody Labeling Kit *Production Scale Optimized for Labeling 1 mg Antibody Per Reaction*
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
| Correction Factor (280 nm) | 0.22 |
| Extinction coefficient (cm -1 M -1) | 406000 |
| Excitation (nm) | 477 |
| Emission (nm) | 678 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
| Overview |
Correction Factor (280 nm) 0.22 | Extinction coefficient (cm -1 M -1) 406000 | Excitation (nm) 477 | Emission (nm) 678 |
Components
Example protocol
AT A GLANCE
1.0 mg Antibody (MW ~150 kDa)
Antibody concentration: 2.0 mg/mL
Antibody volume: 500 µL
PREPARATION OF WORKING SOLUTION
Before opening the vials, warm all components and briefly centrifuge. Immediately prepare necessary solutions before starting conjugation. This protocol is a recommendation.
Prepare a 500 µL antibody solution in PBS with a concentration of 2 mg/mL.
Note: The protein should be dissolved in 1X phosphate-buffered saline (PBS), pH 7.2 - 7.4. If the protein is dissolved in buffers containing primary amines, like Tris and/or glycine, it must be dialyzed against 1X PBS, pH 7.2 - 7.4, or use Amicon Ultra0.5, Ultracel-10 Membrane, 10 kDa (Cat No. UFC501008 from Millipore) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well.
Warm up a vial of Buccutite™ MTA (Component B) to room temperature.
Add 5 µL of DMSO (not provided) to the vial of Buccutite™ MTA (Component B), and mix well by pipetting.
SAMPLE EXPERIMENTAL PROTOCOL
Add 25 µL of Reaction Buffer (Component C) to the antibody solution.
Transfer 5 µL of the reconstituted Buccutite™ MTA DMSO solution into the vial of antibody solution, and mix well by pipetting.
Rotate the reaction mixture at room temperature for 1 hour, then purify using a desalting column.
Invert the provided spin column (Component D) several times to re-suspend the settled gel and remove any bubbles.
Snap off the tip and place the column in a washing tube (2 mL, not provided). Remove the cap to allow the excess packing buffer to drain by gravity to the top of the gel bed.
Note: If the column does not begin to flow, push the cap back into the column and remove it again to start the flow. Discard the drained buffer, and then place the column back into the Washing Tube.
Centrifuge at 1000 x g for 2 minutes in a swinging bucket centrifuge to remove the packing buffer. Then discard the buffer. Refer to the 'Centrifugation Notes' section below for instructions.
Apply 1-2 mL 1X PBS (pH 7.2-7.4) to the column. After each application of PBS, let the buffer drain out by gravity, or centrifuge the column for 2 minutes to remove the buffer. Discard the buffer from the collection tube. Repeat this process for 3-4 times.
Centrifuge at 1000 x g for 2 minutes in a swinging bucket centrifuge to remove the packing buffer. Then discard the buffer. Refer to the 'Centrifugation Notes' section below for instructions.
Place the column into a clean collecting tube (1.5 mL, not provided). Then, take the antibody-Buccutite™ MTA solution from step 3 of the "Run Antibody-Buccutite™ MTA Reaction" section and load it carefully and directly into the center of the column.
After loading the sample, add 40 μL of 1X PBS (pH 7.2-7.4), centrifuge the column for 2 minutes at 1,000 x g, and collect the solution that contains the desired antibody-Buccutite™ MTA solution.
Warm up a vial of Buccutite™ FOL-Activated PerCP (Component A) to room temperature.
Note: Each vial of Buccutite™ FOL-Activated PerCP contains an optimized amount of dye to label 1 mg of IgG (MW ~150 kDa) at 2 mg/mL in PBS, the kit can also be used to label other proteins (>10 kDa).
Make a Buccutite™ FOL-Activated PerCP solution by adding 100 µL of ddH2O into the vial of Buccutite™ FOL-Activated PerCP (Component A), and mix well by pipetting or vortexing.
Add the purified Antibody-Buccutite™ MTA solution directly into the vial of Buccutite™ FOL-Activated PerCP solution. Rotate the mixture for 1-2 hours at room temperature.
The antibody-PerCP conjugate is now ready for immediate use or can be stored at 4°C.
For optimal performance, it is recommended to purify the antibody-PerCP conjugate using size exclusion chromatography (SEC). The following SEC columns are suitable for this purpose: Superdex 200 Increase 100/300 GL (Cytiva) and ENrich™ SEC 650 10 x 300 Column (Bio-Rad).
Spectrum
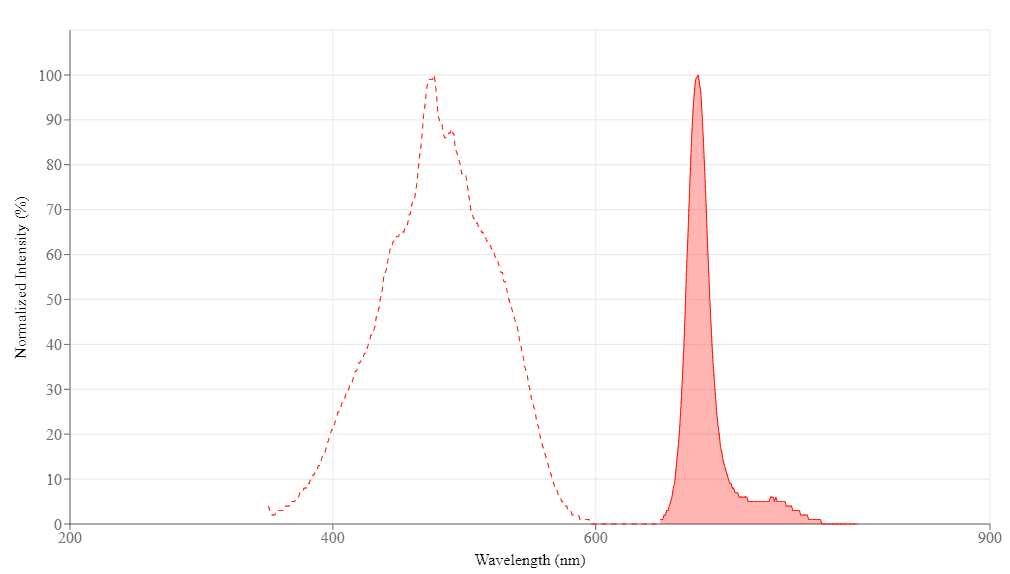
Spectral properties
| Correction Factor (280 nm) | 0.22 |
| Extinction coefficient (cm -1 M -1) | 406000 |
| Excitation (nm) | 477 |
| Emission (nm) | 678 |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (280 nm) |
| Buccutite™ Rapid PE Antibody Labeling Kit *Microscale Optimized for Labeling 100 ug Antibody Per Reaction* | 565 | 574 | 1960000 | 0.82 | 0.175 |
| Buccutite™ Rapid APC Antibody Labeling Kit *Microscale Optimized for Labeling 100 ug Antibody Per Reaction* | 651 | 660 | 730000 | - | 0.195 |
| Buccutite™ Rapid PE Antibody Labeling Kit *Microscale Optimized for Labeling 25 ug Antibody Per Reaction* | 565 | 574 | 1960000 | 0.82 | 0.175 |
| Buccutite™ Rapid APC Antibody Labeling Kit *Microscale Optimized for Labeling 25 ug Antibody Per Reaction* | 651 | 660 | 730000 | - | 0.195 |
| Buccutite™ Rapid PE Antibody Labeling Kit *Production Scale Optimized for Labeling 1 mg Antibody Per Reaction* | 565 | 574 | 1960000 | 0.82 | 0.175 |
References
Authors: Miatello, Jordi and Faivre, Valérie and Marais, Clémence and Raineau, Mégane and Payen, Didier and Tissieres, Pierre
Journal: Cytometry. Part B, Clinical cytometry (2023)
Authors: Zhang, Ting and Chen, Xiao and Chen, Deyong and Wang, Junbo and Chen, Jian
Journal: Frontiers in bioengineering and biotechnology (2023): 1195940
Authors: Watanabe, Eizo and Akamatsu, Toshinobu and Ohmori, Masaaki and Kato, Mayu and Takeuchi, Noriko and Ishiwada, Naruhiko and Nishimura, Rintaro and Hishiki, Haruka and Fujimura, Lisa and Ito, Chizuru and Hatano, Masahiko
Journal: Cytokine (2022): 155723
Authors: Quirós-Caso, Covadonga and Arias Fernández, Tamara and Fonseca-Mourelle, Ariana and Torres, Héctor and Fernández, Luis and Moreno-Rodríguez, Maria and Ariza-Prota, Miguel Ángel and López-González, Francisco Julián and Carvajal-Álvarez, Miguel and Alonso-Álvarez, Sara and Moro-García, Marco Antonio and Colado, Enrique
Journal: Cytometry. Part B, Clinical cytometry (2022)
Authors: Zhang, Linlin and Liu, Mengjia and Zhang, Meng and Ai, Junhong and Tian, Jiao and Wang, Ran and Xie, Zhengde
Journal: Journal of visualized experiments : JoVE (2022)
Authors: Gatti, Arianna and Buccisano, Francesco and Scupoli, Maria T and Brando, Bruno
Journal: Cytometry. Part B, Clinical cytometry (2021): 194-205
Authors: Mandala, Wilson and Harawa, Visopo and Munyenyembe, Alinane and Soko, Monica and Longwe, Herbert
Journal: Current research in immunology (2021): 184-193
Authors: Hsu, Yu-I and Mahara, Atsushi and Yamaoka, Tetsuji
Journal: Peptides (2021): 170470
Authors: Yesillik, Sait and Gupta, Sudhir
Journal: Immunity, inflammation and disease (2020): 441-446
Authors: Takamatsu, Hiroyuki and Yoroidaka, Takeshi and Fujisawa, Momoko and Kobori, Kazuya and Hanawa, Masako and Yamashita, Takeshi and Murata, Ryoichi and Ueda, Mikio and Nakao, Shinji and Matsue, Kosei
Journal: International journal of hematology (2019): 377-381