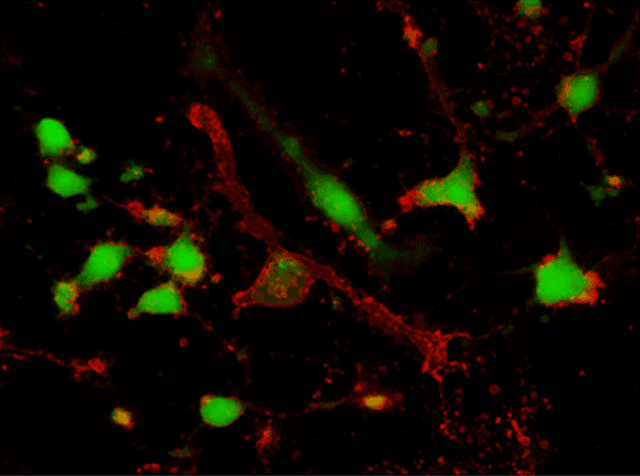
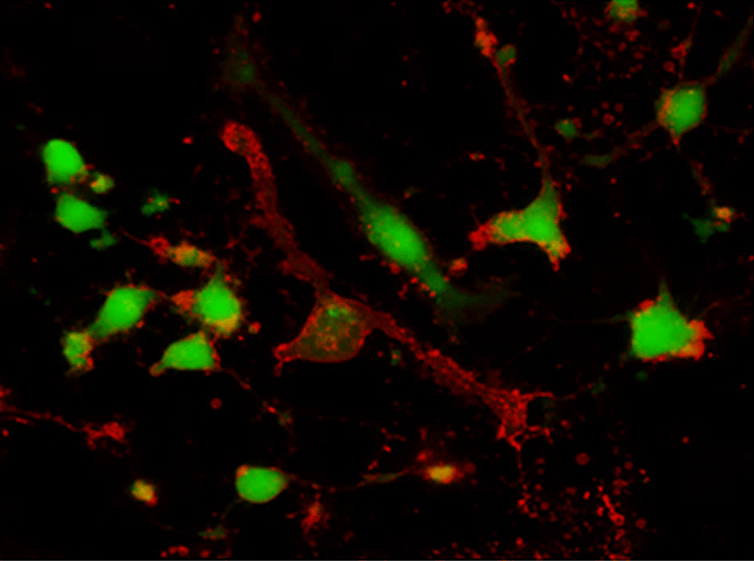
Calcein AM
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 2 to 5 mM stock solution of Calcein AM in high-quality, anhydrous DMSO.
Note: When reconstituted in DMSO, Calcein AM is a clear, colorless solution.
PREPARATION OF WORKING SOLUTION
Prepare a Calcein AM working solution of 1 to 10 µM in the buffer of your choice (e.g., Hanks and Hepes buffer). For most cell lines, Calcein AM at the final concentration of 4 to 5 µM is recommended. The exact concentration of indicators required for cell loading must be determined empirically.
Note: The nonionic detergent Pluronic® F-127 can be used to increase the aqueous solubility of AM esters. In the staining buffer, the final Pluronic® F-127 concentration should be approximately 0.02%. A variety of Pluronic® F-127 products can be purchased from AAT Bioquest. Avoid long-term storage of AM esters in the presence of Pluronic® F-127.
Note: If your cells contain organic anion-transporters, probenecid (1–2.5 mM) or sulfinpyrazone (0.1–0.25 mM) may be added to the working solution to reduce leakage of the de-esterified indicators. A variety of ReadiUse™ Probenecid products, Including water-soluble, sodium salt, and stabilized solutions, can be purchased from AAT Bioquest.
SAMPLE EXPERIMENTAL PROTOCOL
- Prepare cells for imaging.
Remove the cell culture medium and wash cells once with serum-free buffer to remove any remaining media.
Note: Serum in cell culture media may contain esterase activity, which can increase background interference.
- Add Calcein AM working solution to the culture.
- Incubate cells at 37 °C for 30 to 60 minutes.
- Replace the dye working solution with HHBS or buffer of your choice (containing an anion transporter inhibitor, such as 1 mM probenecid, if applicable) to remove any excess probes.
- Measure the fluorescence intensity using either a fluorescence microscope equipped with a FITC filter set, a flow cytometer equipped with a blue laser and a 530/30 nm filter (FITC channel), or a fluorescence plate reader at Ex/Em = 490/525 nm cutoff 515 nm.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 100.517 µL | 502.583 µL | 1.005 mL | 5.026 mL | 10.052 mL |
| 5 mM | 20.103 µL | 100.517 µL | 201.033 µL | 1.005 mL | 2.01 mL |
| 10 mM | 10.052 µL | 50.258 µL | 100.517 µL | 502.583 µL | 1.005 mL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Alternative formats
| Name | Color | Grade |
| Calcein, AM *UltraPure grade* *CAS 148504-34-1* | Green | UltraPure |
| Calcein, AM *UltraPure grade* *CAS 148504-34-1* | Green | UltraPure |
| Calcein, AM *CAS 148504-34-1* | Green | Pure |
| Calcein Red™ AM | Red | - |
| Calcein UltraGreen™ AM | UltraGreen | - |
| Calcein UltraBlue™ AM | UltraBlue | - |
| Calcein Blue, AM *CAS 168482-84-6* | Blue | - |
| Calcein Deep Red™ AM ester | Deep Red | - |
Product family
| Name | Excitation (nm) | Emission (nm) |
| Calcein Blue *CAS 54375-47-2* | 354 | 441 |
Citations
Authors: Zhang, Ying and Xin, Guang and Zhou, Qilong and Yu, Xiuxian and Feng, Lijuan and Wen, Ao and Zhang, Kun and Wen, Tingyu and Zhou, Xiaoli and Wu, Qiuling and others,
Journal: Toxicology and Applied Pharmacology (2024): 116871
Authors: Yang, Zhenzhen and Teng, Yulu and Lin, Meng and Peng, Yiwei and Du, Yitian and Sun, Qi and Gao, Datong and Yuan, Quan and Zhou, Yu and Yang, Yiliang and others,
Journal: ACS nano (2024)
Authors: Tu, Tian and Shi, Yuan and Zhou, Boya and Wang, Xiaoyu and Zhang, Wenjie and Zhou, Guangdong and Mo, Xiumei and Wang, Wenbo and Wu, Jinglei and Liu, Wei
Journal: npj Regenerative Medicine (2023): 67
Authors: Zhu, Jun-Ya and Yao, Wen and Ni, Xi-Sen and Yao, Mu-Di and Bai, Wen and Yang, Tian-Jing and Zhang, Zi-Ran and Li, Xiu-Miao and Jiang, Qin and Yan, Biao
Journal: Cell Reports Medicine (2023)
Authors: Geraets, EKW
Journal: (2023)
References
Authors: Li, Deng and Xiang, Xiaocong and Yang, Fei and Xiao, Dongqin and Liu, Kang and Chen, Zhu and Zhang, Ruolan and Feng, Gang
Journal: Biochemical and Biophysical Research Communications (2017)
Authors: Zibek S, Stett A, Koltay P, Hu M, Zengerle R, Nisch W, Stelzle M.
Journal: Biophys J. (2006)
Authors: Klesius PH, Evans JJ, Shoemaker CA, Pasnik DJ.
Journal: Fish Shellfish Immunol (2006): 20
Authors: Bratosin D, Mitrofan L, Palii C, Estaquier J, Montreuil J.
Journal: Cytometry A (2005): 78
Authors: Schoonen WG, Westerink WM, de Roos JA, Debiton E.
Journal: Toxicol In Vitro (2005): 505