mFluor™ Violet 450 maleimide
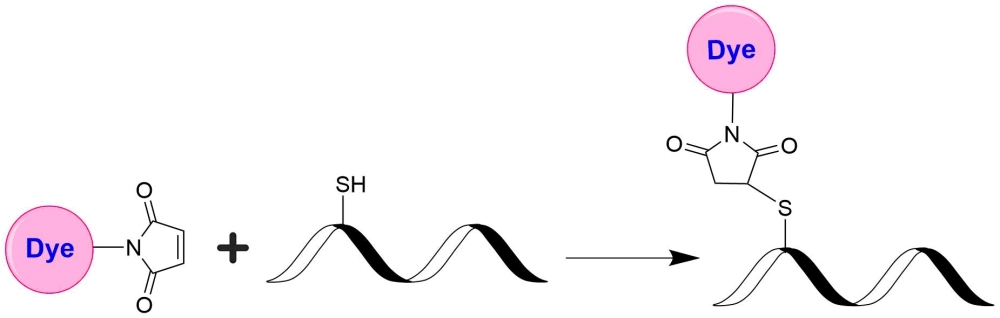
AAT Bioquest's mFluor™ dyes are developed for multicolor flow cytometry-focused applications. These dyes have large Stokes Shifts, and can be well excited by the laser lines of flow cytometers (e.g., 405 nm, 488 nm and 633 nm). mFluor™ Violet 450 dyes have fluorescence excitation and emission maxima of ~405 nm and ~450 nm respectively. These spectral characteristics make them an excellent replacement for Pacific Blue™ labeling dye. mFluor™ Violet 450 maleimide is stable and shows good reactivity and selectivity with thiol group. mFluor™ Violet 450 maleimide provides a convenient tool to label monoclonal, polyclonal antibodies or other proteins (>10 kDa) for flow cytometric applications with the violet laser excitation.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1600 | 1 mg | Price |
Physical properties
| Molecular weight | 664.63 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 406 |
| Correction factor (260 nm) | 0.338 |
| Correction factor (280 nm) | 0.078 |
| Extinction coefficient (cm -1 M -1) | 35000 1 |
| Excitation (nm) | 406 |
| Emission (nm) | 445 |
| Quantum yield | 0.81 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 4, 2026

