FastClick™ Cy7 Azide
Ordering information
| Price | |
| Catalog Number | |
| Unit Size | |
| Quantity |
Additional ordering information
| Telephone | 1-800-990-8053 |
| Fax | 1-800-609-2943 |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Shipping | Standard overnight for United States, inquire for international |
Physical properties
| Molecular weight | 895.11 |
| Solvent | DMSO |
Spectral properties
| Correction Factor (260 nm) | 0.05 |
| Correction Factor (280 nm) | 0.036 |
| Correction Factor (482 nm) | 0.0005 |
| Correction Factor (565 nm) | 0.0193 |
| Correction Factor (650 nm) | 0.165 |
| Extinction coefficient (cm -1 M -1) | 250000 |
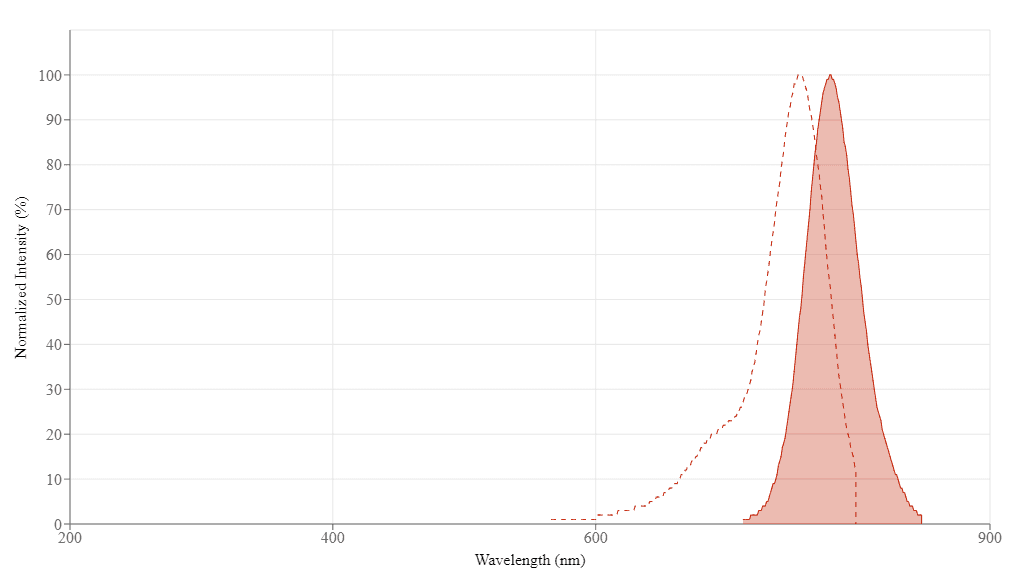
| Excitation (nm) | 756 |
| Emission (nm) | 779 |
| Quantum yield | 0.3 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Alternative formats
| FastClick™ Cy7 Alkyne |
| Overview |
See also: Click Chemistry
Molecular weight 895.11 | Correction Factor (260 nm) 0.05 | Correction Factor (280 nm) 0.036 | Correction Factor (482 nm) 0.0005 | Correction Factor (565 nm) 0.0193 | Correction Factor (650 nm) 0.165 | Extinction coefficient (cm -1 M -1) 250000 | Excitation (nm) 756 | Emission (nm) 779 | Quantum yield 0.3 |
FastClick™ Cy7 Azide contains both the CAG moiety of FastClick (for assisting click efficiency) and Cy7 fluorophore (as the fluorescence tag) for developing Cy7-based fluorescent probes. Cy7 is one of the most widely used near infrared (NIR) fluorophores. It has the identical fluorescence spectra to Alexa Fluor 750, a Cy7 analog. FastClick™ reagents have been developed by the scientists of AAT Bioquest for enhancing the yield and reaction speed of copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. They contain a copper-chelating ligand that significantly stabilizes the Cu(I) oxidation state and thus accelerates the click reaction. They do not require the use of an external copper-chelator (such as the common THPTA or BTTAA). The high concentration of copper chelators is known to have a detrimental effect on DNA/RNA, thus causing biocompatibility issues. The introduction of a copper-chelating moiety at the reporter molecule allows for a dramatic raise of the effective Cu(I) concentration at the reaction site and thus accelerates the reaction. Under extremely mild conditions the FastClick™ azides and alkynes react much faster in high yield compared to the corresponding conventional CuAAC reactions. Click chemistry was developed by K. Barry Sharpless as a robust and specific method of ligating two molecules together. Two important characteristics make click chemistry attractive for assembling biomolecules. First, click reactions are bio-orthogonal, thus the click chemistry-functionalized biomolecules would not react with the natural biomolecules that lack a clickable functional group. Second, the reactions proceed with ease under mild conditions, such as at room temperature and in aqueous media.
Calculators
Common stock solution preparation
Table 1. Volume of DMSO needed to reconstitute specific mass of FastClick™ Cy7 Azide to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 111.718 µL | 558.591 µL | 1.117 mL | 5.586 mL | 11.172 mL |
| 5 mM | 22.344 µL | 111.718 µL | 223.436 µL | 1.117 mL | 2.234 mL |
| 10 mM | 11.172 µL | 55.859 µL | 111.718 µL | 558.591 µL | 1.117 mL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Open in Advanced Spectrum Viewer

Spectral properties
| Correction Factor (260 nm) | 0.05 |
| Correction Factor (280 nm) | 0.036 |
| Correction Factor (482 nm) | 0.0005 |
| Correction Factor (565 nm) | 0.0193 |
| Correction Factor (650 nm) | 0.165 |
| Extinction coefficient (cm -1 M -1) | 250000 |
| Excitation (nm) | 756 |
| Emission (nm) | 779 |
| Quantum yield | 0.3 |
Product Family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| FastClick™ Cy3 Azide | 555 | 569 | 1500001 | 0.151 | 0.07 | 0.073 |
| FastClick™ Cy5 Azide | 651 | 670 | 2500001 | 0.271, 0.42 | 0.02 | 0.03 |
| FastClick™ XFD350 Azide | 343 | 441 | 19000 | - | 0.25 | 0.19 |
| FastClick™ XFD488 Azide | 499 | 520 | 71000 | 0.921 | 0.30 | 0.11 |
| FastClick™ XFD555 Azide | 553 | 568 | 150000 | 0.11 | 0.08 | 0.08 |
| FastClick™ XFD647 Azide | 650 | 671 | 239000 | 0.331 | 0.00 | 0.03 |
| FastClick™ XFD750 Azide | 752 | 776 | 240000 | 0.121 | 0.00 | 0.04 |
Images

Figure 1. FastClick™ Cy7 Azide contains both the moiety of FastClick (for assisting click efficiency) and Cy7 fluorophore (as the fluorescence tag) for developing Cy7-based fluorescent probes. FastClick™ reagents have been developed by the scientists of AAT Bioquest for enhancing the yield and reaction speed of copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction.
![The reaction (Green Bar) of FastClick Cy5 Azide with coumarin alkyne occurs under extremely mild conditions (e.g., [Azide] = 0.02 mM, [Alkyne] = 0.02 mM, [CuSO4] = 0.02 mM, [Sodium Ascorbate] = 5 mM, in 100 mM HEPES) under which the common Cy5 azide does not effectively react with the coumarin alkyne substrate.](/_next/image?url=https%3A%2F%2Fimages.aatbio.com%2Fproducts%2Ffigures-and-data%2Ffastclick-cy7-azide%2Ffigure-for-fastclick-cy7-azide_mQE5U.png&w=3840&q=75)
Figure 2. The reaction (Green Bar) of FastClick Cy5 Azide with coumarin alkyne occurs under extremely mild conditions (e.g., [Azide] = 0.02 mM, [Alkyne] = 0.02 mM, [CuSO4] = 0.02 mM, [Sodium Ascorbate] = 5 mM, in 100 mM HEPES) under which the common Cy5 azide does not effectively react with the coumarin alkyne substrate.
References
View all 20 references: Citation Explorer
Fluorescent Polymer-AS1411-Aptamer Probe for dSTORM Super-Resolution Imaging of Endogenous Nucleolin.
Authors: Fabre, Laura and Rousset, Corentin and Monier, Karine and Da Cruz-Boisson, Fernande and Bouvet, Philippe and Charreyre, Marie-Thérèse and Delair, Thierry and Fleury, Etienne and Favier, Arnaud
Journal: Biomacromolecules (2022): 2302-2314
Authors: Fabre, Laura and Rousset, Corentin and Monier, Karine and Da Cruz-Boisson, Fernande and Bouvet, Philippe and Charreyre, Marie-Thérèse and Delair, Thierry and Fleury, Etienne and Favier, Arnaud
Journal: Biomacromolecules (2022): 2302-2314
Miktoarm Star Polymers with Environment-Selective ROS/GSH Responsive Locations: From Modular Synthesis to Tuned Drug Release through Micellar Partial Corona Shedding and/or Core Disassembly.
Authors: Lotocki, Victor and Yazdani, Hossein and Zhang, Qiaochu and Gran, Evan Rizzel and Nyrko, Anastasiia and Maysinger, Dusica and Kakkar, Ashok
Journal: Macromolecular bioscience (2021): e2000305
Authors: Lotocki, Victor and Yazdani, Hossein and Zhang, Qiaochu and Gran, Evan Rizzel and Nyrko, Anastasiia and Maysinger, Dusica and Kakkar, Ashok
Journal: Macromolecular bioscience (2021): e2000305
Coumarin-grafted blue-emitting fluorescent alginate as a potentially valuable tool for biomedical applications.
Authors: Araújo, Marco and Bidarra, Sílvia J and Alves, Pedro M and Valcarcel, Jesús and Vázquez, José A and Barrias, Cristina C
Journal: Journal of materials chemistry. B (2020): 813-825
Authors: Araújo, Marco and Bidarra, Sílvia J and Alves, Pedro M and Valcarcel, Jesús and Vázquez, José A and Barrias, Cristina C
Journal: Journal of materials chemistry. B (2020): 813-825
In cell Gd3+-based site-directed spin labeling and EPR spectroscopy of eGFP.
Authors: Kucher, Svetlana and Korneev, Sergej and Klare, Johann P and Klose, Daniel and Steinhoff, Heinz-Jürgen
Journal: Physical chemistry chemical physics : PCCP (2020): 13358-13362
Authors: Kucher, Svetlana and Korneev, Sergej and Klare, Johann P and Klose, Daniel and Steinhoff, Heinz-Jürgen
Journal: Physical chemistry chemical physics : PCCP (2020): 13358-13362
Kiss and Run: Promoting Effective and Targeted Cellular Uptake of a Drug Delivery Vehicle Composed of an Integrin-Targeting Diketopiperazine Peptidomimetic and a Cell-Penetrating Peptide.
Authors: Feni, Lucia and Parente, Sara and Robert, Clémence and Gazzola, Silvia and Arosio, Daniela and Piarulli, Umberto and Neundorf, Ines
Journal: Bioconjugate chemistry (2019): 2011-2022
Authors: Feni, Lucia and Parente, Sara and Robert, Clémence and Gazzola, Silvia and Arosio, Daniela and Piarulli, Umberto and Neundorf, Ines
Journal: Bioconjugate chemistry (2019): 2011-2022
Mechanochemical Activation of Fluorogenic CuAAC "Click" Reactions for Stress-Sensing Applications.
Authors: Michael, Philipp and Biewend, Michel and Binder, Wolfgang H
Journal: Macromolecular rapid communications (2018): e1800376
Authors: Michael, Philipp and Biewend, Michel and Binder, Wolfgang H
Journal: Macromolecular rapid communications (2018): e1800376
Dendrimer-Based Signal Amplification of Click-Labelled DNA in Situ.
Authors: Raddaoui, Nada and Stazzoni, Samuele and Möckl, Leonhard and Viverge, Bastien and Geiger, Florian and Engelke, Hanna and Bräuchle, Christoph and Carell, Thomas
Journal: Chembiochem : a European journal of chemical biology (2017): 1716-1720
Authors: Raddaoui, Nada and Stazzoni, Samuele and Möckl, Leonhard and Viverge, Bastien and Geiger, Florian and Engelke, Hanna and Bräuchle, Christoph and Carell, Thomas
Journal: Chembiochem : a European journal of chemical biology (2017): 1716-1720
Synthesis of bifunctional molecules containing [12]aneN3 and coumarin moieties as effective DNA condensation agents and new non-viral gene vectors.
Authors: Yue, Pan and Zhang, Ying and Guo, Zhi-Fo and Cao, Ao-Cheng and Lu, Zhong-Lin and Zhai, Yong-Gong
Journal: Organic & biomolecular chemistry (2015): 4494-505
Authors: Yue, Pan and Zhang, Ying and Guo, Zhi-Fo and Cao, Ao-Cheng and Lu, Zhong-Lin and Zhai, Yong-Gong
Journal: Organic & biomolecular chemistry (2015): 4494-505
Asymmetric AB3 Miktoarm Star Polymers: Synthesis, Self-Assembly, and Study of Micelle Stability Using AF4 for Efficient Drug Delivery.
Authors: Moquin, Alexandre and Sharma, Anjali and Cui, Yiming and Lau, Anthony and Maysinger, Dusica and Kakkar, Ashok
Journal: Macromolecular bioscience (2015): 1744-54
Authors: Moquin, Alexandre and Sharma, Anjali and Cui, Yiming and Lau, Anthony and Maysinger, Dusica and Kakkar, Ashok
Journal: Macromolecular bioscience (2015): 1744-54
Site-specific conjugation of 8-ethynyl-BODIPY to a protein by [2 + 3] cycloaddition.
Authors: Albrecht, Marcel and Lippach, Andreas and Exner, Matthias P and Jerbi, Jihene and Springborg, Michael and Budisa, Nediljko and Wenz, Gerhard
Journal: Organic & biomolecular chemistry (2015): 6728-36
Authors: Albrecht, Marcel and Lippach, Andreas and Exner, Matthias P and Jerbi, Jihene and Springborg, Michael and Budisa, Nediljko and Wenz, Gerhard
Journal: Organic & biomolecular chemistry (2015): 6728-36
![The reaction (Green Bar) of FastClick Cy5 Azide with coumarin alkyne occurs under extremely mild conditions (e.g., [Azide] = 0.02 mM, [Alkyne] = 0.02 mM, [CuSO4] = 0.02 mM, [Sodium Ascorbate] = 5 mM, in 100 mM HEPES) under which the common Cy5 azide does not effectively react with the coumarin alkyne substrate.](/_next/image?url=https%3A%2F%2Fimages.aatbio.com%2Fproducts%2Ffigures-and-data%2Ffastclick-cy7-azide%2Ffigure-for-fastclick-cy7-azide_mQE5U.png&w=128&q=25)