Click Chemistry
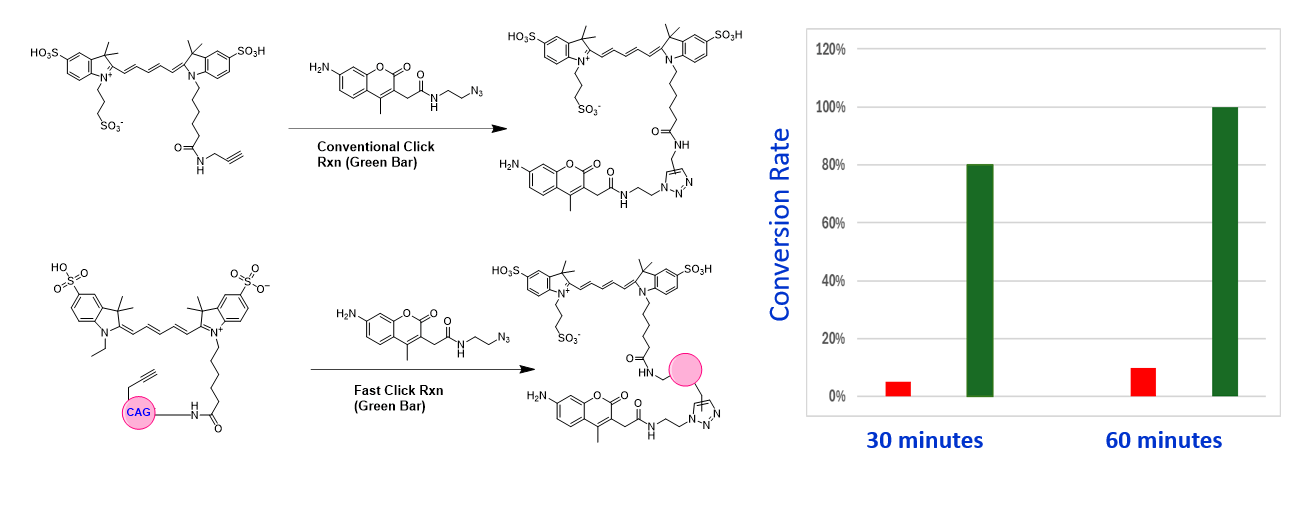
The reaction (Green Bar) of FastClick™ Cy5 Alkyne with coumarin azide occurs under extremely mild conditions (e.g., [Azide] = 0.02 mM, [Alkyne] = 0.02 mM, [CuSO4] = 0.02 mM, [Sodium Ascorbate] = 5 mM, in 100 mM HEPES) under which the common Cy5 alkyne does not effectively react with the coumarin azide substrate.
Click chemistry reactions are stereospecific, simple to perform and can be conducted in easily removable or benign solvents. This concept was developed in parallel with the interest within the pharmaceutical, material, and other industries in capabilities of generating large libraries of compounds for screening in discovery research. Several types of reaction have been identified that fulfill these criteria. They are thermodynamically-favored reactions that lead specifically to one product, such as nucleophilic ring opening reactions of epoxides and aziridines; non-aldol type carbonyl reactions, such as formation of hydrazones and heterocycles; eletrophilic additions to carbon-carbon multiple bonds, such as oxidative formation of epoxides and Michael Additions; and cycloaddition reactions.
Click Chemistry Reactive Labels
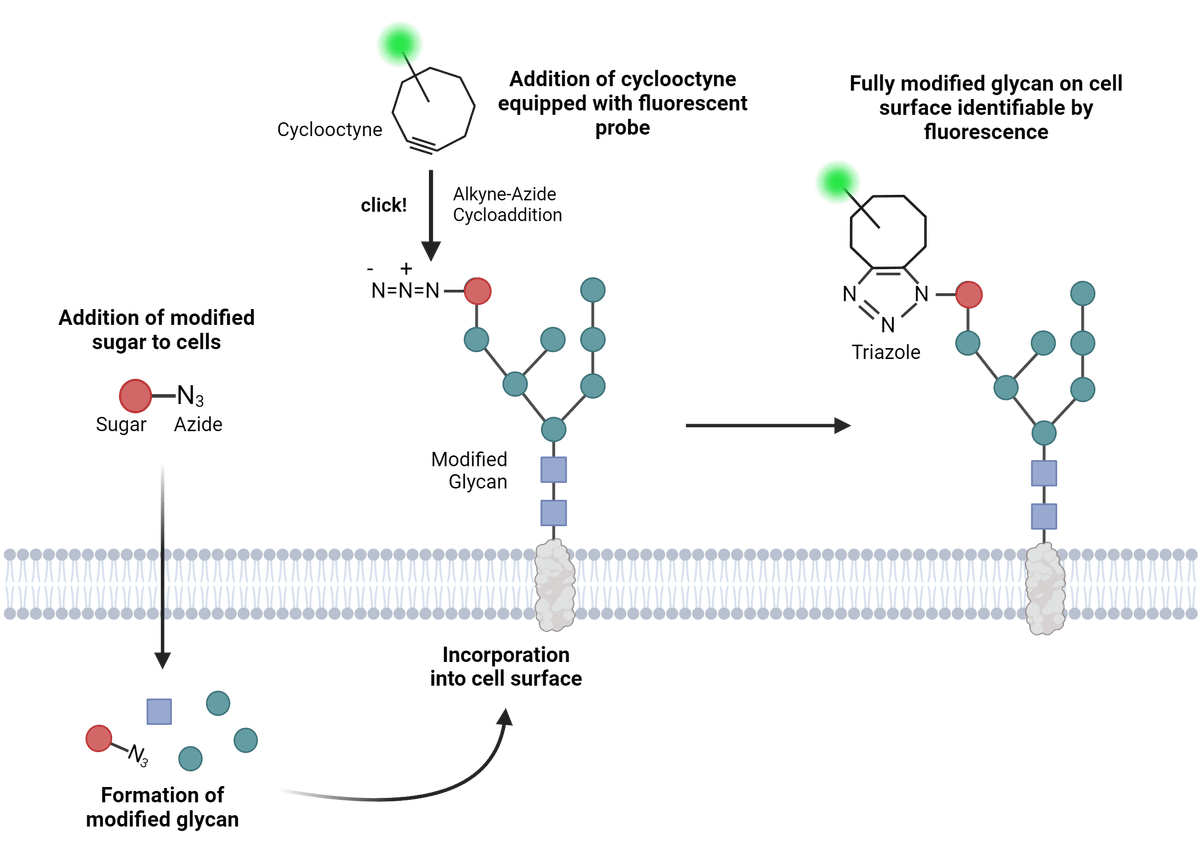
The five major steps of click chemistry bioconjugation. This particular example is of conjugated molecules that have been integrated into the cells, typically via incubation or other techniques. The cycloaddition reaction is between the azide and alkyne on either the labeled probe or the target biomolecule. The example above shows the eventual visualization of the targeted cell surface protein via a fluorescence-labeled click probe. Figure made in BioRender.
AAT Bioquest offers a variety of dye azides and alkynes for labeling peptides and oligonucleotides. These clickable reagents include both common fluorescent dyes (e.g., fluoresceins, rhodamines and cyanines) and non-fluorescent quenchers. Our Tide Fluor™ and Tide Quencher™ dyes are specifically optimized for preparing novel FRET substrates.
Table 1. Dye Azides potentially suitable for labeling of DNA oligos by Click Chemistry.
| Dye ▲ ▼ | Superior Alternative to ▲ ▼ | Excitation Max (nm) ▲ ▼ | Emission Max (nm) ▲ ▼ | Unit Size ▲ ▼ | Cat No. ▲ ▼ |
| Tide Fluor™ 1 azide [TF1 azide] | EDANS | 341 | 448 | 5 mg | 2236 |
| Tide Fluor™ 2 azide [TF2 azide] | Fluoresceins (FAM and FITC) | 503 | 525 | 1 mg | 2252 |
| Tide Fluor™ 3 azide [TF3 azide] | Cy3® | 554 | 578 | 1 mg | 2254 |
| Tide Fluor™ 4 azide [TF4 azide] | ROX/Texas Red® | 578 | 602 | 1 mg | 2300 |
| Tide Fluor™ 5WS azide [TF5WS azide] | Cy5® | 649 | 664 | 1 mg | 2275 |
| Tide Fluor™ 6WS azide [TF6WS azide] | Cy5.5 | 682 | 701 | 1 mg | 2302 |
| Tide Fluor™ 7WS azide [TF7WS azide] | Cy7® | 756 | 780 | 1 mg | 2304 |
| Tide Fluor™ 8WS azide [TF8WS azide] *Near Infrared Emission* | IRDye® 800 | 785 | 801 | 1 mg | 2306 |
| Assaywise Letters: |
Optimized FastClick™ Click Reagents

FastClick™ Biotin Alkyne contains both the CAG moiety of FastClick (for assisting click efficiency) and biotin (as the detection tag) for developing biotin-based probes. It readily reacts with an azido-containing biomolecule under extremely mild conditions.
Table 3. FastClick™ Series
| Cat# ▲ ▼ | Product Name ▲ ▼ | Unit Size ▲ ▼ |
| 72700 | FastClick™ Cy3 Azide | 1 mg |
| 72702 | FastClick™ Cy5 Azide | 1 mg |
| 72704 | FastClick™ Cy7 Azide | 1 mg |
| 72710 | FastClick™ 5-FAM Azide | 1 mg |
| 72711 | FastClick™ 6-FAM Azide | 1 mg |
| 72712 | FastClick™ 5-TAMRA Azide | 1 mg |
| 72713 | FastClick™ 6-TAMRA Azide | 1 mg |
| 72714 | FastClick™ 6-ROX Azide | 1 mg |
| 72730 | FastClick™ XFD350 Azide | 1 mg |
| 72733 | FastClick™ XFD405 Azide | 1 mg |
| Services: |
References
Metabolically active bacteria detected with click chemistry in low organic matter rainwater
Aqueous synthesis of a small-molecule lanthanide chelator amenable to copper-free click chemistry
Mechanochemical Activation of Fluorogenic CuAAC “Click” Reactions for Stress-Sensing Applications
Site-specific conjugation of 8-ethynyl-BODIPY to a protein by [2 + 3] cycloaddition