Cal-520®, potassium salt
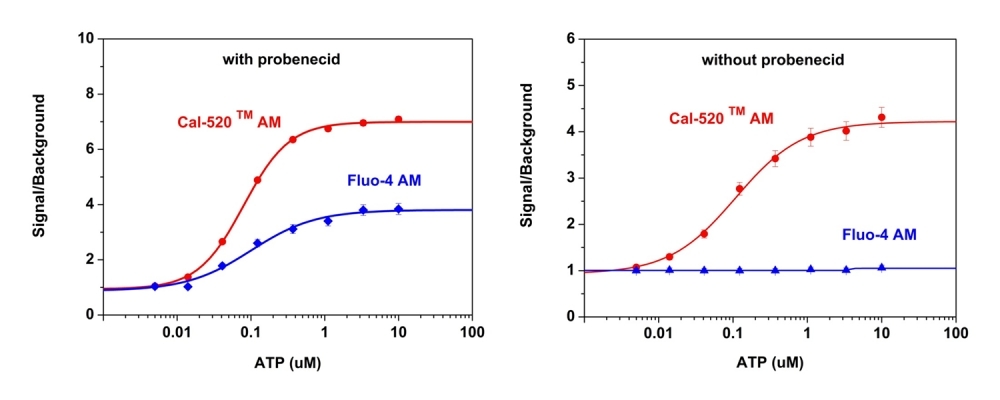
Cal-520® provides a robust homogeneous fluorescence-based assay tool for detecting intracellular calcium mobilization. Cal-520® AM is a new fluorogenic calcium-sensitive dye with a significantly improved signal to noise ratio and intracellular retention compared to the existing green calcium indicators (such as Fluo-3 AM and Fluo-4 AM). Cells expressing a GPCR or calcium channel of interest that signals through calcium can be preloaded with Cal-520® AM which can cross cell membrane. Once inside the cell, the lipophilic blocking groups of Cal-520® AM are cleaved by esterases, resulting in a negatively charged fluorescent dye that stays inside cells. Its fluorescence is greatly enhanced upon binding to calcium. When cells stimulated with agonists, the receptor signals the release of intracellular calcium, which significantly increase the fluorescence of Cal-520®. The characteristics of its long wavelength, high sensitivity, and >100 times fluorescence enhancement, make Cal-520® AM an ideal indicator for the measurement of cellular calcium. The high S/N ratio and better intracellular retention make the Cal-520® calcium assay a robust tool for evaluating GPCR and calcium channel targets as well as for screening their agonists and antagonists. Cal-520® sodium or potassium salt is the hydrolyzed salt of Cal-520® AM in cells. It selectively binds to calcium ion, and has the fluorescence that is strongly dependent on calcium concentration.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21140 | 10x50 ug | Price | |
| 21141 | 1 mg | Price |
Physical properties
| Dissociation constant (Kd, nM) | 320 |
| Molecular weight | 921.08 |
| Solvent | Water |
Spectral properties
| Excitation (nm) | 492 |
| Emission (nm) | 515 |
| Quantum yield | 0.75 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 20, 2026

