Cell Meter™ Annexin V Apoptosis Assay Kit
Example protocol
AT A GLANCE
Prepare cells with test compounds (200 µL/sample).
Add Annexin V-iFluor® 594 assay solution.
Incubate at room temperature for 30 - 60 minutes.
Analyze cells using a flow cytometer with a 610/20 nm filter (PE-Texas Red channel) or fluorescence microscope with a TRITC or Texas Red filter set.
CELL PREPARATION
For guidelines on cell sample preparation, please visit:
https://www.aatbio.com/resources/guides/cell-sample-preparation.html
SAMPLE EXPERIMENTAL PROTOCOL
Treat cells with test compounds for a desired period of time (4 - 6 hours for Jurkat cells treated with staurosporine) to induce apoptosis.
Centrifuge the cells to get 1 - 5 × 105 cells/tube.
Resuspend cells in 200 µL of Assay Buffer (Component B).
Add 2 µL of Annexin V-iFluor® 594 (Component A) into the cells.
Incubate at room temperature for 30 to 60 minutes, protected from light.
Add 300 µL of Assay Buffer (Component B) to increase volume before analyzing the cells with a flow cytometer or fluorescence microscope.
Monitor the fluorescence intensity using a flow cytometer with a 610/20 nm filter (PE-Texas Red channel) or a fluorescence microscope with a TRITC or Texas Red filter set.
Quantify Annexin V- iFluor® 594 binding using a flow cytometer with 610/20 nm filter (PE-Texas Red channel).
Note: Annexin V binding flow cytometric analysis on adherent cells is not routinely tested since specific membrane damage may occur during cell detachment or harvesting. However, methods for utilizing Annexin V for flow cytometry on adherent cell types have been previously reported by Casciola-Rosen et al. and van Engelend et al.
Pipette the cell suspension after incubation, rinse 1 - 2 times with Assay Buffer, and then resuspend the cells with assay buffer.
Add the cells on a glass slide that is covered with a glass cover-slip.
Note: For adherent cells, it is recommended to grow the cells directly on a cover-slip. After incubation with Annexin V-iFluor® 594, rinse 1 - 2 times with Assay Buffer, and add Assay Buffer back to the cover-slip. Invert the cover-slip on a glass slide and visualize the cells. The cells can also be fixed in 2% formaldehyde after incubation with Annexin V-iFluor® 594 and visualized under a microscope.
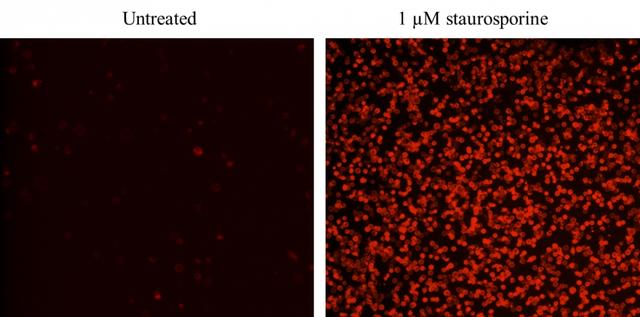
Analyze the apoptotic cells with Annexin V-iFluor® 594 under a fluorescence microscope using a TRITC or Texas Red filter set. The orange staining on the plasma membrane indicates the Annexin V-iFluor® 594 binding to PS on the cell surface.
Spectrum
Alternative formats
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Green Fluorescence Optimized for Flow Cytometry* | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Orange Fluorescence Optimized for Flow Cytometry* | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Deep Red Fluorescence Optimized for Flow Cytometry* | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
Citations
Authors: Zeng, Weiquan and Wu, Meizhu and Cheng, Ying and Liu, Liya and Han, Yuying and Xie, Qiurong and Li, Jiapeng and Wei, Lihui and Fang, Yi and Chen, Youqin and others,
Journal: Plos one (2022): e0279851
Authors: Lin, Yung-Kai and Sharma, Ruchi and Ma, Hsu and Chen, Wen-Shyan and Yao, Chao-Ling
Journal: Journal of the Taiwan Institute of Chemical Engineers (2017)
Authors: Do, Duyen Thi and Phan, Nam Nhut and Wang, Chih-Yang and Sun, Zhengda and Lin, Yen-Chang
Journal: Cytotechnology (2016): 2589--2604
Authors: Maggiorani, Damien and Dissard, Romain and Belloy, Marcy and Saulnier-Blache, Jean-S{\'e}bastien and Casemayou, Audrey and Ducasse, Laure and Gr{\`e}s, Sandra and Belli{\`e}re, Julie and Caubet, C{\'e}cile and Bascands, Jean-Loup and others,
Journal: PLoS One (2015): e0131416
Authors: Maggiorani, Damien and Dissard, Romain and Belloy, Marcy and Saulnier-Blache, Jean-Sébastien and Casemayou, Audrey and Ducasse, Laure and Grès, S and ra , undefined and Bellière, Julie and Caubet, Cécile and Basc, undefined and s, Jean-Loup and others, undefined
Journal: PloS one (2015): e0131416
References
Authors: Kurschus FC, Pal PP, Baumler P, Jenne DE, Wiltschi B, Budisa N.
Journal: Cytometry A (2009): 626
Authors: Wang CY, Lin YS, Su WC, Chen CL, Lin CF.
Journal: Mol Biol Cell (2009): 4153
Authors: Palma PF, Baggio GL, Spada C, Silva RD, Ferreira SI, Treitinger A.
Journal: Braz J Infect Dis (2008): 108
Authors: Hu T, Shi J, Jiao X, Zhou J, Yin X.
Journal: Braz J Med Biol Res (2008): 750
Authors: Chen S, Cheng AC, Wang MS, Peng X.
Journal: World J Gastroenterol (2008): 2174