Cal-520® AM, a Development Overview
A History of Calcium Indicators
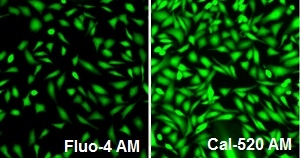
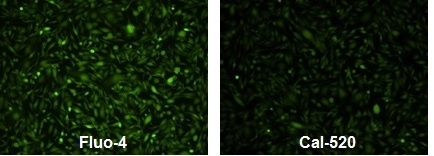
Responses of endogenous P2Y receptor to ATP in CHO-M1 cells with probenecid. CHO-M1 cells were seeded overnight at 40,000 cells per 100 µL per well in a Costar 96-well black wall/clear bottom plate. 100 µL of 4 µM Fluo-4 AM (left, Cat# 20552), Cal 520™ AM (right, Cat# 21130) in HHBS was added into the wells, and the cells were incubated at 37 °C for 2 hours. The dye loading medium was replaced with 100 µL HHBS and 50 µL of 300 µM ATP were added. The cells were imaged with a fluorescence microscope (Olympus IX71) using FITC channel.
The first big breakthrough in calcium imaging came in the 1980s, when a team of scientists at the University of California Berkeley developed the first fluorescein-based calcium indicator. Their research paved the way for now well-established calcium probes, such as Fluo-3 AM and Fluo-4 AM. These hydrophobic, fluorescein-based AM esters readily pass through the membranes of living cells, where once inside, they are rapidly hydrolyzed by esterases and set to detect calcium. Upon successful binding with calcium, Fluo-3 or Fluo-4 will become fluorescent, emitting a signal at ~520 nm when excited by a ~490 nm light source.
Problems Abound
A significant achievement in its own right, these first generation fluorescein-based dyes did however suffer from several drawbacks. Most notably, these calcium probes were significantly affected by high background fluorescence, also known as noise. In general, researchers like to minimize background fluorescence as it creates many problems for bioassays and data analysis. It can obscure significant results and hide true positives while generating false signals, making interpretation of experimental responses incredibly difficult.
The severity of the background fluorescence generated by Fluo-3 AM and Fluo-4 AM has led to intense investigation into the matter, with researchers keying in on two likely causes. The first has to do with the chemical properties of acetoxymethyl (AM) esters. In solution, AM esters like Fluo-3 AM and Fluo-4 AM tend to hydrolyze, cleaving off the acetoxymethyl group. This means that when it comes time to load the dye into cells, a significant portion of the probe will be impermeable as they do not contain the AM functional group which allows them to pass through the cell membrane. This results not only in a lower fluorescence signal (as less dye permeated the cell), but also a higher background fluorescence as well. This is because the impermeable Fluo-3 or Fluo-4 outside the cell will still fluoresce and contribute to an elevated baseline fluorescence.
The second major cause of high background fluorescence is postulated to result from leakage of dyes through organic anion transporters. It is assumed that Fluo-3 and Fluo-4 are poorly localized in the cytosol and can actually be transported into intracellular organelles or the extracellular matrix after being loaded into cells. This results in a situation similar to that of the hydrolyzed AM esters. That is, Fluo-3 or Fluo-4 will collect outside of the cell and fluoresce, again leading to an elevated baseline fluorescence. This is a particularly serious problem with calcium flux assays and calcium imaging as both depend on geospatial quantification of calcium. If the calcium probe itself is poorly localized and is being transported about, these bioassays become harder, if not impossible, to interpret.
The Past Solution
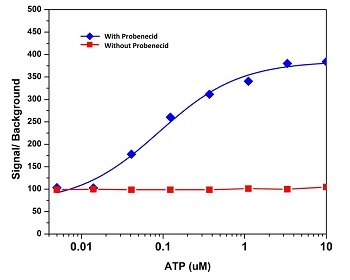
ATP-stimulated calcium response of endogenous P2Y receptor in CHO-K1 cells measured with Fluo-4 AM (Cat# 20552). CHO-K1cells were seeded overnight at 50,000 cells per 100 µL per well in a 96-well black wall/clear bottom Costar plate. 100 µL of 5 mM Fluo-4 AM with (blue) or without (red) 2.5 mM probenecid was added into the cells, and the cells were incubated at 37 °C for 1 hour. ATP (50 µL/well) was added by FlexStation® (Molecular Devices) to achieve the final indicated concentrations.
Probenecid, as a drug, currently has two major uses. The first is to treat gout and hyperuricemia. The second is to prolong the effects of other drugs, such as penicillin. In both cases, the postulated mechanism of action is the same. That is, probenecid is hypothesized to target the kidneys wherein it inhibits organic anion transporter activity. This in turn causes greater retention of select compounds, such as pharmaceutical drugs, thereby prolonging their effects. This mechanism of action also explains the benefit to using probenecid in conjunction with Fluo-3 and Fluo-4. As you may recall, Fluo-3 and Fluo-4 can leak out of cells due to organic anion transporter activity. By inhibiting those transporters, probenecid leads to better Fluo-3 and Fluo-4 retention and consequently, lower background fluorescence.
While probenecid was a good patchwork solution, we did not however find it a very satisfactory one. This was because we saw the benefits of probenecid usage as simultaneously its biggest flaw. That is, probenecid is a drug and, by its very nature, it alters cellular function. On the one hand, this means better cellular retention of calcium indicators. On the other, however, it means there is a whole host of yet unknown effects probenecid may have on cellular function (eg. increased cytotoxicity), leading to possible confounding variables. Furthermore, it introduces the need for additional controls (eg. probenecid dose), making it inconvenient to use.
Our Development Process
Unsatisfied with the industry solution, we decided to seek our own to the background fluorescence problem. To this end, our process included three steps.
First, we conceptualized the solution and created an actionable goal. This involved our highly experienced chemists designing various alterations to the structure of Fluo-3 and Fluo-4. For example, one approach was to amend chemical properties to increase the stability of the AM ester bond. This would minimize hydrolysis in solution and improve cell loading. A second approach was to use functional groups to improve probe water solubility. By changing the chemical substituents, we sought to enhance cellular retention and improve localization. After a great deal of theoretical modeling, we were able to zero in on a few key structural changes that would solve the problem of high background fluorescence.
The next two steps will be familiar to anyone who has engaged in chemical production. It is the dual question of synthesis and scale. For us as a company, the question was not only how to produce our designed compound, but how to produce our designed compound efficiently. While optimal synthesis and scaling are both challenging and extremely hot topics of study in chemistry, it suffices to say that our teams of expert chemists were able to work out a reaction path with good yield. This was critical to our development process as good reaction paths drive down production costs and allow us to pass the savings to our customers. Combined with our strict Quality Management System, our efficient manufacturing process allows us to provide the highest quality products at the best values. This is a policy which we emphasize for all of our products, but particularly so during the development of our new calcium indicator. In the end, such a policy proved ideal. Our new calcium indicator, Cal-520® has been extremely successful in reducing background fluorescence, with customers reporting results that have even exceeded our own expectations.
Background comparison of Fluo-4 AM (Cat# 20552) and Cal-520® AM (Cat# 21130). CHO-K1 cells were seeded overnight at 50,000 cells per 100 µL per well in a 96-well black wall/clear bottom Costar plate. 100 µL of 5 uM Fluo-4 AM or Cal-520® AM with probenecid was added into the cells and incubated at 37 °C for 1 hour. After incubation, cells were washed once with HHBS and then background was imaged without ATP treatment.
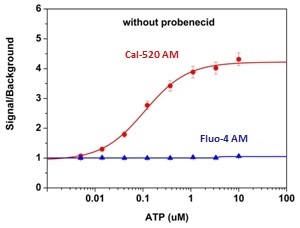
ATP-stimulated calcium responses of endogenous P2Y receptors in CHO-K1 cells incubated with Cal-520® AM (red curve, Cat# 21130), or Fluo-4 AM (blue curve, Cat# 20552) respectively, without probenecid under the same conditions. CHO-K1 cells were seeded overnight at 50,000 cells/100 µL/well in a Costar black wall/clear bottom 96-well plate. 100 µL of 5 µM Fluo-4 AM or Cal-520® AM in HHBS was added into the cells, and the cells were incubated at 37 °C for 2 hours. ATP (50 µL/well) was added using FlexSation® to achieve the final indicated concentrations.
Features Aplenty
By making alterations to chemical structure, we significantly improved the stability of the AM ester bond. In AM ester form, Cal-520® AM shows very minimal spontaneous hydrolysis in solution when compared with Fluo-3 AM and Fluo-4 AM.
Furthermore, we have optimized Cal-520®'s chemical structure so that it is better localized in cells. Unlike Rhod-2, which localizes in mitochondria, Cal-520® predominantly localizes in the cytosol. Additionally, once localized, it retains very well, even without the addition of probenecid. This is in huge contrast to Fluo-3 and Fluo-4. These features taken together mean lower background fluorescence and significantly improved signal to background ratio. Again, this is without the addition of probenecid or the need for constant washing of cells.
Furthermore, Cal-520® maintains the convenient spectra of Fluo-3 and Fluo-4. This allows for easy transition from older, outdated calcium indicators. There is no need to redesign experiment or purchase new equipment.
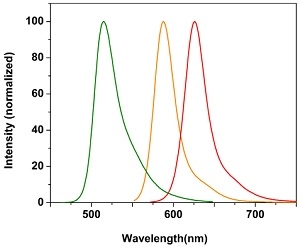
Normalized emission spectra of Cal-520® (Green), Cal-590™ (Orange) and Cal-630™ (Red) Cal-630™ (Red).
Finally, we are excited to announce that we have adapted Cal-520® AM for easy application in multiplexing. For multiplexing, we have Cal-590™ AM and Cal-630™ AM, longer wavelength excitations that are completely compatible with green fluorescence indicators like GFP while maintaining the high signal to background ratio of Cal-520® AM.
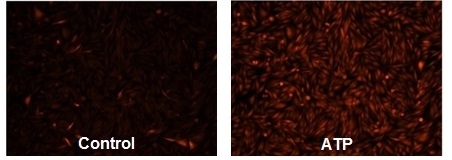
Responses of endogenous P2Y receptor to ATP in CHO-K1 cells. CHO-K1 cells were seeded overnight at 40,000 cells per 100 µL per well in a Costar 96-well black wall/clear bottom plate. 100 µL of 4 µM Cal-590™ AM (Cat# 20510) in HHBS with 1 mM probenecid were added into the wells, and the cells were incubated at 37 °C for 2 hours. The dye loading mediums were replaced with 100 µL HHBS and 1 mM probenecid, then imaged with a fluorescence microscope (Olympus IX71) using TRITC channel before and after adding 50 µL of 300 µM ATP .
Table 1. Spectral Comparison of Fluo-3, Fluo-4, Fluo-8® and Cal-520
| Dye ▲ ▼ | Ex (nm) ▲ ▼ | Em (nm) ▲ ▼ | QY* ▲ ▼ | Kd (nM) ▲ ▼ |
| Cal-520™ | 492 | 514 | 0.75 | 320 |
| Fluo-3 | 506 | 525 | 0.15 | 390 |
| Fluo-4 | 493 | 515 | 0.16 | 345 |
| Fluo-8® | 490 | 514 | 0.16 | 389 |
- *QY = Fluorescence Quantum Yield in the presence of 5 mM calcium citrate.
Applications and Beyond
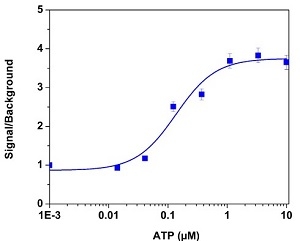
ATP-stimulated calcium response of endogenous P2Y receptor in CHO-K1 cells measured with Cal-630™ AM (Cat# 20530). CHO-K1 cells were seeded overnight at the cell density of 50,000 cells per 100 µL per well in a 96-well black wall/clear bottom plate. 100 µL of 10 µg/mL Cal-630™ AM with 2.0 mM probenecid was added into the cells, and the cells were incubated at 37 °C for 2 hours. ATP (50 µL/well) was added by FlexStation® (Molecular Devices) to achieve the final indicated concentrations.
As Cal-520® grows in popularity, we have seen it come to be cited in papers on a regular basis and in a variety of different fields, from neurobiology to cell signaling to cardiology. In particular, we are excited about the research currently being done by Tada et al. on monitoring single action potentials from neocortical neurons in vivo. We are also closely watching the research by Lock et al. on their investigation of long distance Ca2+ communication via microtubules and regulation by inositol triphosphate (IP3). We are happy to see the adoption of Cal-520® by so many researchers, and we hope that Cal-520® will continue to be a powerful tool for researchers interested in studying intracellular calcium. For more information on Cal-520®, please click here or email for inquiry at info@aatbio.com.
Table 2. Single Wavelength Fluorescent Calcium Indicators
| Cat No. ▲ ▼ | Product Name ▲ ▼ | Ex (nm) ▲ ▼ | Em (nm) ▲ ▼ | Kd ▲ ▼ | Unit Size ▲ ▼ |
| 21140 | Cal-520®, potassium salt | 492 | 514 | 320 nM | 10x50 µg |
| 21144 | Cal-520FF™, potassium salt | 492 | 514 | 9.8 nM | 10x50 µg |
| 20518 | Cal-590™, potassium salt | 573 | 588 | 561 nM | 5x50 µg |
| 20538 | Cal-630™, potassium salt | 608 | 626 | 792 nM | 5x50 µg |
| 20500 | Cal Green™ 1, hexapotassium salt | 506 | 531 | 190 nM | 10x50 µg |
| 21017 | Fluo-3, pentapotassium salt | 506 | 526 | 390 nM | 1 mg |
| 21019 | Fluo-3FF, pentapotassium salt | 506 | 526 | 10 µM | 1 mg |
| 20556 | Fluo-4, Pentapotassium Salt | 494 | 516 | 345 µM | 10x50 µg |
| 21089 | Fluo-8®, potassium salt | 494 | 517 | 389 µM | 10x50 µg |
| 21102 | Fluo-8FF™, potassium salt | 494 | 517 | 10 µM | 10x50 µg |